Practice Essentials
Bacteremia is the presence of viable bacteria in the circulating blood. Most episodes of occult bacteremia spontaneously resolve, particularly those caused by Streptococcus pneumoniae and Salmonella, and serious sequelae are increasingly uncommon. However, serious bacterial infections occur, including pneumonia, septic arthritis, brain abscesses, osteomyelitis, cellulitis, meningitis, and sepsis, possibly resulting in death.
Signs and symptoms
The significance of the history in a febrile child varies according to the patient’s age. Elements of the history include the following:
-
Duration of fever (overall, inadequate for clinical identification of occult bacteremia)
-
History that indicates a specific illness
-
History that indicates risk for occult bacteremia (eg, Rochester criteria)
-
History of an underlying medical condition
-
History of prematurity
-
History of another reason for an increased temperature (eg, recent vaccinations, overbundling, or environmental exposure to heat involving a young infant)
-
History of gastroenteritis (suggestive of possible Salmonella bacteremia)
-
Epidemiology
-
Risk factors for invasive pneumococcal disease
Physical examination may include the following:
-
Assessment of general appearance
-
Assessment of vital signs (temperature, pulse, respiratory rate, blood pressure) – The risk of bacteremia has consistently been found to increase with increases in temperature; however, studies have shown a variation in risk at given temperatures based on age
-
Assessment of response to antipyretics
-
Inspection for signs of focal infection of the skin, soft tissue, bone, or joints
-
Inspection for petechiae
-
Evaluation for acute otitis media or upper respiratory tract infection
-
Evaluation for pneumonia
-
Evaluation for recognizable viral infections
See Presentation for more detail.
Diagnosis
Laboratory studies that may be helpful in the workup for possible bacteremia include the following:
-
White blood cell (WBC) count – At present, this is the current established standard screen for bacterial infection, though other screening tests may yield equal or superior results
-
Absolute neutrophil count (ANC)
-
Absolute band count (ABC) – This is not recommended as a screen for occult bacteremia but is used in some guidelines as part of the low-risk criteria
-
Erythrocyte sedimentation rate (ESR) – This is not currently recommended as a screening test for occult bacteremia
-
C-reactive protein (CRP) level – Although it is not currently an established standard screening test for occult bacteremia, CRP level screening of febrile children in the emergency department is a part of the established protocol at numerous medical centers
-
Cytokine (eg, interleukin [IL]-1, IL-6, and tumor necrosis factor-a [TNF-a]) levels – These tests have not been thoroughly investigated, have marginal clinical utility, and are of unknown cost-effectiveness; they are not recommended as routine screening laboratory studies for occult bacteremia
-
Procalcitonin level – This appears to be more sensitive and more specific for bacterial infection than are other laboratory values currently used as screening tests and has good results in illnesses of short duration
-
Urinalysis and urine culture
-
Stool studies for children with diarrhea (eg, for Salmonella)
-
Plasma clearance rate (for meningococcal bacteremia)
-
Lumbar puncture and cerebrospinal fluid (CSF) analysis
-
Blood culture
The only imaging study routinely used in infants and children with fever without source (FWS) is chest radiography to evaluate for pneumonia if the child has tachypnea or crackles are heard. Pneumonia should be considered in febrile children with no other source of infection.
See Workup for more detail.
Management
Most infants and young children who are evaluated for occult bacteremia present with a fever. While the child is evaluated to determine a source of the fever, fever reduction with medication is reasonable and is widely accepted.
A combination of age, temperature, and screening laboratory test results is used to determine the risk for serious bacterial infection or occult bacteremia. Subsequent management depends on the level of risk, as follows:
-
Low-risk children are generally monitored as outpatients
-
Children who do not fit low-risk criteria are treated with empiric antibiotics either as inpatients or as outpatients
The choice of empiric antibiotic treatment is primarily based on the likely causes of bacteremia for a given patient (which are related to age) and the likelihood of resistance. Regimens include the following:
-
Neonates younger than 28 days – Ampicillin plus gentamicin; ampicillin plus cefotaxime or ceftriaxone (unless hyperbilirubinemia is present); third-generation cephalosporins are not currently recommended as single-agent therapy in this population
-
Infants aged 1-3 months – Ampicillin plus gentamicin; ampicillin plus cefotaxime; ceftriaxone; whether Listeria coverage is required in this population is controversial
-
Infants and children aged 3-36 months – Ceftriaxone (most commonly)
Treatment algorithms that have been employed include the following:
-
Kuppermann approach (1999) [1]
-
Baraff approach (2000) [2]
-
Nigrovic and Malley management guideline (2004) [3]
Further inpatient care may include the following:
-
Hospitalization – This is recommended for all febrile infants younger than 28 days pending culture results; for infants aged 1-3 months who do not meet low-risk criteria; and for children aged 3-36 months if sepsis is a concern or if outpatient treatment is not feasible
-
Tailored antibiotic therapy
Further outpatient care may include the following:
-
Close observation and reevaluation in 24 hours
-
Antibiotic treatment at follow-up
-
Monitoring of blood cultures
-
Reevaluation if the blood cultures become positive with a known pathogen, followed by appropriate treatment
The image below illustrates a treatment approach in febrile infants younger than 3 months.
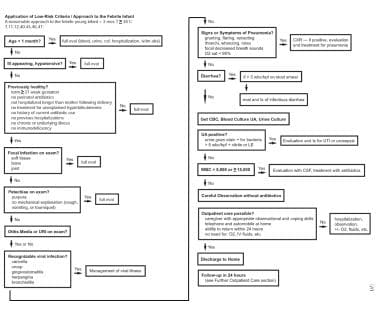 Application of low-risk criteria and approach for the febrile infant: A reasonable approach for treating febrile infants younger than 3 months who have a temperature of greater than 38°C.
Application of low-risk criteria and approach for the febrile infant: A reasonable approach for treating febrile infants younger than 3 months who have a temperature of greater than 38°C.
See Treatment and Medication for more detail.
Background
Bacteremia is the presence of viable bacteria in the circulating blood. [4] This may or may not have any clinical significance because harmless, transient bacteremia may occur following dental work or other minor medical procedures; however, this bacteremia is generally clinically benign and self-resolving in children who do not have an underlying illness or immune deficiency or a turbulent cardiac blood flow. The concern with occult bacteremia is that it could progress to a more severe local or systemic infection if left untreated. Most episodes of occult bacteremia spontaneously resolve, and serious sequelae are increasingly uncommon. However, serious bacterial infections occur, including pneumonia, septic arthritis, osteomyelitis, cellulitis, meningitis, brain abscesses, and sepsis, possibly resulting in death. [1, 5]
With the development and widespread use of effective vaccines to the common serious bacterial infections of infancy (Haemophilus influenzae type B and Streptococcus pneumoniae), the rate of infectious caused by these pathogens has dramatically declined. Many of the studies in children with occult bacteremia were done prior to the introduction of one or both of these vaccines and, as such, may overestimate the likelihood of occult bacteremia.
Patients with occult bacteremia by definition do not have clinical evidence other than fever (a systemic response to infection). [6] First described in the 1960s in young febrile children with unsuspected pneumococcal infection, bacteremia is defined as the presence of bacteria in the bloodstream of a febrile child who was previously healthy; the child does not clinically appear to be ill and has no apparent focus of infection. [7, 8] Occult bacteremia has been defined as bacteremia not associated with clinical evidence of sepsis (shock or purpura) or toxic appearance, underlying significant chronic medical conditions, or clear foci of infection (other than acute otitis media) upon examination in a patient who is discharged and sent home after an outpatient evaluation. [1]
Often, the only manifestation of occult bacteremia is fever or a minor infection (eg, otitis media, upper respiratory tract infection). [6] Therefore, in a busy clinic or emergency department, infants and young children with occult bacteremia are difficult to distinguish from others in the waiting-room.
Fever is common in pediatric patients. Children average 4-6 fevers by age 2 years. [9] Fever also prompts many visits to the pediatric clinic and emergency department. Approximately 8-25% of doctor's visits by children younger than 3 years are for fever [2, 6, 9, 10] ; 65% of children younger than 3 years visit a physician for acute febrile illness. [10, 11]
Fever is less common in infants younger than 3 months than in those aged 3 months to 3 years. Young infants may not mount a fever response and may also be hypothermic in response to illness or stress. [9] Approximately 1% of infants younger than 2 months present with fever, and fever is twice as common in infants aged 1-2 months as it is in newborns younger than 1 month. [9]
Of all pediatric patients presenting for evaluation of fever, 20% have fever for which the source of infection is undetermined after a history and physical examination. [2] Of all infants and young children who present to the hospital for any reason, 1.6% appear nontoxic, were previously healthy, are older than 3 months, and have a fever without a source (FWS). [2]
Bacteremia may also occur in children with focal infections or in children who have sepsis (ie, clinical evidence other than fever of a systemic response to infection). Children with sepsis generally appear ill, have an increased heart rate or respiratory rate and may have a change in temperature (typically fever, although hypothermia is often seen in very young infants and newborns). Severe sepsis results in hypotension, hypoperfusion, or organ dysfunction. Septic shock occurs in children who do not respond to adequate volume resuscitation or require vasopressors or inotropes. Although bacteria may be present in the bloodstream of children with focal infections, sepsis, severe sepsis, or septic shock, the focus of this article is occult bacteremia.
Pathophysiology
Much of the pathophysiology of occult bacteremia is not fully understood. The presumed mechanism begins with bacterial colonization of the respiratory passages or other mucosal surface; bacteria may egress into the bloodstream of some children because of host-specific and organism-specific factors. Once viable bacteria have gained access to the bloodstream, they may be spontaneously cleared, they may establish a focal infection, or the infection may progress to septicemia; the possible sequelae of septicemia include shock, disseminated intravascular coagulation, multiple organ failure, and death. [6, 12]
Often, fever is the only presenting sign in patients with occult bacteremia and is defined as increased temperature caused by resetting the thermoregulatory center in the hypothalamus by action of cytokines. [9] The cytokines may be produced in response to viral or bacterial pathogens or by immune complexes. An increased temperature does not always represent a fever. Hyperthermia may also be due to increased heat production as occurs in exercise or decreased heat loss as occurs in overbundling, neither of which involves resetting of the hypothalamic thermostat.
Chase et al found that a number of clinical variables (respiratory failure, vasopressor use, neutrophilia, bandemia, thrombocytopenia, indwelling venous catheter, abnormal temperature, suspected line or urinary infection, or endocarditis) were predictive of bacteremia in a sample of 5630 emergency department patients with suspected infection. [13]
A child's immune system helps determine which bacteria gain initial access to the bloodstream, whether bacteremia spontaneously resolves or progresses to serious bacterial illness, and whether cytokines are produced to mount a fever response. The risk of life-threatening bacterial disease is greatest in young infants when their immune system is least mature; they have poor immunoglobulin G (IgG) antibody response to encapsulated bacteria and decreased opsonin activity, macrophage function, and neutrophil activity. [14, 15]
Clearly, some children are more susceptible to bacterial infection, which may initially be uncomplicated bacteremia but could rapidly lead to more serious complications. Immunosuppression due to neoplastic disease or its treatment or defects in antibody responses or neutrophil responses predispose certain children to invasive infection. Bacteremia should be considered, with a low threshold for evaluation and treatment, in patients with impaired immunity or invasive medical devices such as indwelling central venous lines.
The pathogens implicated in occult bacteremia change in response to vaccination against the common pathogenic strains. These changes govern the choices for empiric therapy of suspected bacteremia.
Etiology
Causes of occult bacteremia vary depending on the age of the infant or child. Very young infants most commonly acquire infections from the mother during childbirth. As a patient's age increases, a gradual shift occurs toward community-acquired infections.
Table 1. Causes of Occult Bacteremia in Neonates and Infants with a Temperature of 38°C or Higher [14, 15, 16, 17, 18] (Open Table in a new window)
Age |
Organism* |
Positive Blood Cultures, % |
Neonates < 1 mo |
Group B Streptococcus |
73 |
Escherichia coli |
8 |
|
S pneumoniae |
3 |
|
Staphylococcus aureus |
3 |
|
Enterococcus species |
3 |
|
Enterobacter cloacae |
3 |
|
Infants aged 1-2 mo |
Group B Streptococcus |
31 |
E coli |
20 |
|
Salmonella species |
16 |
|
S pneumoniae |
10 |
|
H influenzae type b |
6 |
|
S aureus |
4 |
|
E cloacae |
4 |
|
* Also, less frequently (< 1%), Listeria species, Klebsiella species, group A Streptococcus, Staphylococcus epidermis, Streptococcus viridans, and N meningitidis |
||
Older infants and children are at risk for bacteremia due to colonization of the nasopharynx or community-acquired organisms. Hib conjugate vaccine has decreased the prevalence of invasive Hib disease by 90% or more in industrialized countries. [2] With the disappearance of Hib as a cause of occult bacteremia in children, the relative frequency of S pneumoniae increased in some medical centers to more than 90%. [19] Since the introduction and widespread use of the pneumococcal vaccines, the rate of vaccine-specific strains has dropped considerably, leading to significant changes in the patterns of causative organisms in more recent studies.
Table 2. Causes of Occult Bacteremia and Changes Over Time in Children Aged 3-36 Months with FWS [1, 6, 10, 12, 20, 21, 22] (Open Table in a new window)
Organism* |
1975-1993, % |
1993, % |
1993-1996, % |
1990 to present, % |
S pneumoniae |
83-86 |
93 |
92 |
89 |
H influenzae type b |
5-13 |
2 |
0 |
0 |
N meningitidis |
1-3 |
… |
… |
… |
Salmonella species |
1-7 |
… |
… |
… |
* Also, less frequently (< 1%), E coli, S aureus, Streptococcus pyogenes, group B Streptococcus, Moraxella species, Kingella species, Yersinia species, and Enterobacter species |
||||
The prevalence of occult bacteremia caused by pneumococcus has greatly decreased since the introduction of the 7-valent conjugate pneumococcal vaccine, which was designed to cover 98% of the strains of S pneumoniae responsible for occult bacteremia. [23] A multicenter surveillance found that 82-94% of S pneumoniae invasive disease was caused by isolates that are contained in the 7-valent conjugate pneumococcal vaccine. [24] Rates of heptavalent vaccine-serotype invasive pneumococcal infection postlicensure have dropped by 56%-100%, depending on location and age. [25, 26, 27, 28]
S pneumoniae types 4, 6B, 9V, 14, 18C, 19F, and 23F are 98% covered by the 7-valent conjugate pneumococcal vaccine. The pattern of serotypes isolated from patients has undergone considerable change since the introduction of the pneumococcal vaccines. In the first few years of use, the number of cases decreased; more recently, the number of reports of nonvaccine strains replacing vaccine strains as causes of invasive pneumococcal infection has increased. In particular, strain 19A is a drug-resistant strain that has been highlighted in several studies, along with serotypes 15 and 33. [28, 29, 30]
Prevnar 13 includes serotypes 1, 3, 5, 6A, 7F, and 19A in addition to those already in Prevnar, and is expected to further reduce the rate of pneumococcal disease.
A study from the Kaiser Permanente group looked at 4255 blood cultures from neonates. They found a significant pathogen in 2% of cultures, of which 56% were E coli, 21% were group B streptococci, and 8% were S aureus, with other infections making up lower percentages. They found no N meningitidis or Listeria monocytogenes infections and only one case of Enterococcus. [31]
Epidemiology
United States data
The risk of bacteremia has been studied by categorizing infants and young children based on age, appearance, temperature, laboratory criteria, numerous low-risk criteria based on a combination of these factors, and past medical history. These studies are part of an ongoing attempt to decide which children require evaluation and treatment and which children can be safely observed without intervention.
Numerous investigators have loosely and specifically defined the terms toxic and lethargic (see Physical Examination). A child who is toxic or lethargic is generally described as making poor eye contact; having poor interactions with parents and the environment; and showing signs on global assessment of poor perfusion, hypoventilation or hyperventilation, or cyanosis. [10]
In children younger than 3 months who have not received complete Haemophilus influenzae type b (Hib) and pneumococcal vaccines, the risk of bacteremia is 1.2-2% in infants who are not toxic and 10-11% in infants who are toxic. [10, 16] In children aged 3-36 months who are toxic, the risk of bacteremia or serious bacterial infection ranges from 10-90%, depending on criteria. [10, 11]
A study by Greenhow et al studied 57,733 blood cultures of a population of children 3 to 36 months old in order to compare the incidence of bacteremia in in the time period pre-7-valent pneumococcal conjugate vaccine (PCV7), post-PCV7/pre-13-valent pneumococcal conjugate vaccine (PCV13), and post-PCV13. The study reported that post-PCV13, Streptococcus pneumoniae bacteremia decreased from 74.5 to 10 to 3.5 per 100,000 children per year (a 95% reduction). With the decrease in pneumococcal rates, Escherichia coli, Salmonella spp, and Staphylococcus aureus rates increased as the leading causes of bacteremia accounting for 77% of cases. [32]
In a retrospective review of positive blood culture results of 181 cases of bacteremia in 177 febrile infants aged 90 days or younger, Biondi and colleagues found that Escherichia coli was the most common causative pathogen, accounting for 42% of cases, followed by group B Streptococcus (23%). [33] Streptococcus pneumoniae was more common in older infants (P = 0.01). No cases of Listeria monocytogenes were identified. [33]
Most studies designed to determine the relationship between temperature and risk of occult bacteremia define fever as a temperature of at least 38°C (100.4°F) in infants younger than 3 months and at least 39°C (102.2°F) in children aged 3-36 months. Because these studies were designed to predict occult bacteremia, they include children who have only FWS, which is defined as an acute febrile illness in which the etiology is not apparent after history is obtained and a careful physical examination is performed. [11]
Numerous studies published in the early 1990s found that 2-15% of febrile infants younger than 3 months had bacteremia. [14, 15, 17, 18] These studies also determined that the risk of occult bacteremia in children aged 3-36 months with FWS was 2.5-11%. [2, 6, 10, 20, 34] According to studies performed after the introduction of the conjugate Hib vaccine, the risk of occult bacteremia was 1.5-2.3% in children aged 3-36 months with FWS. [23, 21, 35] A recent study from the Kaiser Permanente group gave a risk of 2.2%, but the majority of positive cultures were judged to be contaminants (247 contaminants vs 93 pathogens out of 4255 cultures), giving a true bacteremia rate of 2%. [31]
Current data indicates an even lower incidence of bacteremia at 1 in 200 (0.5%) in febrile children who have been fully immunized. [36] These recent data have questioned the need to obtain blood cultures in nontoxic children with fever.
Clinical trials and postlicensure studies suggest that the 7-valent conjugate pneumococcal vaccine is 90% effective in preventing invasive disease caused by Streptococcus pneumoniae. Widespread use has significantly decreased the overall risk of occult bacteremia, especially with regards to vaccine-specific strains of streptococcus. [2, 37, 24]
The appearance of the nonvaccine pneumococcus strain 19A, which has been responsible for some particularly invasive (and drug-resistant) infections, is a concern. This is discussed in more detail below, and it is expected that the widespread use of the 13-valent conjugate pneumococcal vaccine will help control this.
International data
According to the World Health Organization, at least 6 million children die each year of pneumococcal infections (eg, pneumonia, meningitis, bacteremia); most of these fatalities occur in developing countries. [38]
Race-, sex-, and age-related demographics
Race
Studies of the prevalence of bacteremia in children in diverse settings have identified no racial, geographic, or socioeconomic predisposition. [6, 8, 12, 39] However, antibiotic resistance patterns vary in different geographic regions, which may affect the treatment of children with bacteremia.
Sex
No sex-based difference in the prevalence or course of bacteremia is known. [12]
Age
Studies of occult bacteremia focus on children younger than 3 years. Some studies show that age does not affect the risk of developing occult bacteremia, [12] whereas other analyses have found that variations in age-based risk depend on the infecting organism.
As noted earlier, in a retrospective review of positive blood culture results of 181 cases of bacteremia in 177 febrile infants aged 90 days or younger, E coli was the most common causative pathogen (42%), followed by group B Streptococcus (23%). [33] Streptococcus pneumoniae was more common in older infants.
Pneumococcal bacteremia is observed in children of all ages; however, children aged 6 months to 2 years are at an increased risk. [1, 8, 21] The prevalence of pneumococcal meningitis peaks in infants aged 3-5 months. Meningococcal bacteremia occurs most frequently in infants aged 3-12 months; the highest risk of meningococcal meningitis is in infants aged 3-5 months. [1, 12] The risk of Salmonella bacteremia is greatest in infants younger than 1 year, especially in those younger than 2 months. [1]
A seasonal variation in febrile children presenting for evaluation is recognized. The peak is from late fall to early spring in children of all ages and is likely because of respiratory and GI viral infections. Another peak occurs during the summer in infants younger than 3 months and is likely due to enteroviral infections and thermoregulation during hot weather. [9] However, most studies do not specifically address seasonal variation associated with bacteremia.
Prognosis
Most episodes of occult bacteremia spontaneously resolve, and serious sequelae are increasingly uncommon. However, serious bacterial infections occur, including pneumonia, septic arthritis, osteomyelitis, cellulitis, meningitis, and sepsis; death may result. [1, 5]
Evaluation, treatment, and follow-up of febrile infants and young children at risk for occult bacteremia significantly decrease the risk for serious bacterial infections and sequelae.
Morbidity/mortality
The natural history, morbidity, and mortality associated with occult bacteremia alone are not clearly understood. In prospective studies of occult bacteremia, although many children were initially observed untreated, all were given antibiotics once blood culture findings became positive for known bacterial pathogens. [40] The widespread adoption of vaccines to the most common childhood bacteria pathogens (H influenzae and S pneumoniae) have further complicated assessment because contemporary data are not directly comparable to historical studies.
In studies performed before the introduction of the Hib conjugate vaccine, children with untreated bacteremia had an 18-21% risk of developing persistent bacteremia and a 2-15% risk of developing important focal infections such as meningitis. [6, 10, 11, 22]
Because widespread use of the Hib vaccine has virtually eliminated invasive Hib disease in the developed world, recent reviews, analyses, and studies have focused on invasive S pneumoniae disease. [41] Children with occult pneumococcal bacteremia have a 6-17% risk of persistent bacteremia, a 2-5.8% risk of meningitis, and a 6-10% risk of other focal complications. [1, 6, 10, 11, 35, 42]
Of all focal infections that develop because of pneumococcal bacteremia, pneumococcal meningitis carries the highest risk for significant morbidity and mortality, including a 25-30% risk of neurologic sequelae such as deafness, intellectual disability, seizures, and paralysis. [2, 40] The mortality rate of pneumococcal meningitis is 6.3-15%, and the overall mortality rate of pneumococcal bacteremia is 0.8%. [2, 24, 40]
Neisseria meningitidis also causes bacteremia in infants and young children. Although the prevalence of meningococcal bacteremia is much lower than that of pneumococcal disease (see Etiology), the morbidity and mortality rates are much greater. Children with meningococcal bacteremia have a 42-50% risk of developing meningitis; a 50% risk of developing serious bacterial infection such as septic shock, pneumonia, and neurologic changes; a 3% risk of developing extremity necrosis; and an overall mortality rate of 4%. [2, 6, 40]
When untreated, Salmonella bacteremia carries a 50% risk of persistent bacteremia and can cause meningitis, sepsis, and death in infants younger than 3 months or in persons who are debilitated or immunocompromised. [1] However, in previously healthy children aged 3-36 months, the risk of meningitis or serious bacterial infection following Salmonella bacteremia is low. [6]
A study by McMullan et al that analyzed the epidemiology of Staphylococcus aureus bacteremia in 1153 children and adolescents from Australia and New Zealand found that the mortality rate in children with bacteremia due to methicillin-susceptible S aureus (MSSA) treated with vancomycin was 14% (6 of 43) compared to 2.6% (22 of 851) in children who were received other medications. [43, 44]
Complications
Potential complications include the following:
-
Complications of bacteremia
Occult bacteremia results in morbidity and mortality due to focal infections that arise following the initial bloodstream infection. Most episodes of occult bacteremia spontaneously resolve, and serious sequelae are increasingly uncommon. However, serious bacterial infections occur, including pneumonia, septic arthritis, osteomyelitis, cellulitis, meningitis, and sepsis; death may result. [1, 5]
Of all focal infections that develop because of pneumococcal bacteremia, pneumococcal meningitis carries the highest risk for significant morbidity and mortality, including a 25-30% risk of neurologic sequelae such as deafness, intellectual disability, seizures, and paralysis. [1, 2]
-
Complications of hospitalization
In addition to complications associated with bacteremia and its sequelae, numerous possible complications are associated with evaluation and empiric treatment of infants and young children at risk for occult bacteremia.
A study of hospitalized febrile infants younger than 2 months found that complications were common, many complications were preventable, and most infants were hospitalized longer than necessary. [45] In this study, 20% of all admissions resulted in at least one complication, and 60% of these complications were believed to be preventable (eg, medications overdose, fluid overload, intravenous infiltrate, intravenous skin sloughing, a kidnapped infant [a preventable complication of hospitalization in general, unrelated to the reason for admission], culture contamination that required follow-up). Of the infants in this study who were evaluated and found not to have bacterial disease based on cultures negative for known bacterial pathogens, 98% were hospitalized longer than 72 hours.
The risk of complications should be considered when weighing the risks and benefits of evaluation and empiric treatment of febrile infants and young children at risk for occult bacteremia and its sequelae. Because the overall risk of occult bacteremia decreases with widespread use of the conjugate pneumococcal vaccine, this balance between risk and benefit may need to be reevaluated.
-
Application of low-risk criteria and approach for the febrile infant: A reasonable approach for treating febrile infants younger than 3 months who have a temperature of greater than 38°C.
-
Application of algorithms for children aged 3-36 months: A reasonable approach for treating infants and young children aged 3-36 months who have a temperature of at least 39.5°C.