Overview
Background
Neonatal resuscitation skills are essential for all health care providers who are involved in the delivery of newborns. The transition from fetus to newborn requires intervention by a skilled individual or team in approximately 10% of all deliveries.
This figure is concerning because 81% of all babies in the United States are born in nonteaching, nonaffiliated level I or II hospitals. In such hospitals, the volume of delivery service may not be perceived as sufficient economic justification for the continuous in-hospital presence of personnel with high-risk delivery room experience, as recommended by the American Academy of Pediatrics (AAP) and the American College of Obstetricians and Gynecologists (ACOG). [1]
Perinatal asphyxia and extreme prematurity are the 2 complications of pregnancy that most frequently necessitate complex resuscitation by skilled personnel. However, only 60% of asphyxiated newborns can be predicted ante partum. The remaining newborns are not identified until the time of birth. Additionally, approximately 80% of low-birth-weight infants require resuscitation and stabilization at delivery.
Nearly one half of newborn deaths (many of which involve extremely premature infants) occur during the first 24 hours after birth. Many of these early deaths also have a component of asphyxia or respiratory depression as an etiology. For the surviving infants, effective management of asphyxia in the first few minutes of life may influence long-term outcome.
Even though prenatal care can identify many potential fetal difficulties ante partum, allowing maternal transfer to the referral center for care, many women who experience preterm labor are not identified prospectively and therefore are not appropriately transferred to a tertiary perinatal center. Consequently, many deliveries of extremely premature infants occur in smaller hospitals.
For this reason, all personnel involved in delivery room care of the newborn should be trained adequately in all aspects of neonatal resuscitation. Additionally, equipment that is appropriately sized to resuscitate infants of all gestational ages should be available in all delivering institutions, even if the institution does not care for preterm or intensive care infants.
Along with the necessary skills, the practitioner should approach any resuscitation with a good comprehension of transitional physiology and adaptation, as well as an understanding of the infant's response to resuscitation. Resuscitation involves much more than possessing an ordered list of technical skills and having a resuscitation team; it requires excellent assessment skills and a grounded understanding of physiology.
This article reviews the adaptation process at delivery, outlines the steps necessary to resuscitate neonates, serves as a review for practitioners who already resuscitate infants, and highlights special problems and controversies. New practitioners must complete the Neonatal Resuscitation Program (NRP) or some other program that introduces resuscitation material and allows skill assessment. After reading the material and practicing the skills, they should work with experienced personnel before providing resuscitation at deliveries.
For patient education resources, see the Public Health Center, as well as Cardiopulmonary Resuscitation (CPR).
Transition to Extrauterine Physiology
To decrease neonatal morbidity and mortality, the practitioner must be able to rapidly identify infants whose transition from an intrauterine to extrauterine physiology is delayed. Neonatal transition requires spontaneous breathing and successful cardiopulmonary changes, as well as other changes to independent organ system functions. A thorough understanding of normal transitional physiology leads to a better understanding of the needs of the infant who is experiencing difficulties and thus should result in a more effective resuscitative effort.
Respiratory adaptation
In utero, most of the blood flow is shunted away from the lungs and directed to the placenta where fetoplacental gas exchange occurs. After birth, the airways and the alveoli must be cleared of fetal lung fluid so that the lungs can operate as a functional respiratory unit providing adequate gas exchange. Pulmonary blood flow must increase, and spontaneous respirations must be established. Fetal pulmonary vascular resistance is high, and the fetal systemic vascular resistance is low. Within minutes of delivery, the newborn's pulmonary vascular resistance may decrease 8- to 10-fold, causing a corresponding increase in neonatal pulmonary blood flow. At birth, the lungs must transition rapidly to become the site for gas exchange, or else cyanosis and hypoxia will rapidly develop.
Accordingly, an understanding of the structure and function of the fetal pulmonary vasculature and the subsequent transition to neonatal physiology is important for facilitating the necessary adaptations during resuscitation. In utero, the lungs develop steadily from early in gestation (see Table 1 below). Knowledge of the stages of development clarifies why neonates born before about 23-24 weeks' gestational age often lack sufficient lung development for survival because of the absence of a capillary network adjacent to the immature ventilatory units.
Table 1. Embryologic Stages of Lung Development (Open Table in a new window)
Stage |
Gestational Age |
Structure Development |
Embryonic |
5 wk |
Bronchi develop, and airway branching occurs; pulmonary veins return to left atrium |
Pseudoglandular |
5-17 wk |
Lungs take on glandular appearance, and there is continual branching of tracheal bronchial tree (ending at 18-19 wk gestation); blood vessels and lymphatics begin to form, and diaphragm develops |
Canalicular |
13-25 wk |
Rich vascular supply develops, and capillaries are brought closer to airways; primitive respiratory bronchioles begin to form |
Terminal air sac |
24-40 wk |
Alveoli appear and begin increasing in number, and blood-gas interface develops; type II alveolar cells appear between 20 and 25 wk and start producing surfactant between 24 and 25 wk, though normal intra-airway concentrations are not reached until ~34 wk |
Postnatal |
40 wk to 8 y |
Thinning of alveolar sac linings and continued alveolar proliferation occur |
Fetal pulmonary physiology
At term, the fetal lung is filled with approximately 20 mL of fluid. Fetal airways, alveoli, and terminal saccules are open and stable at normal fetal lung volumes, distended by lung fluid secreted by the pulmonary epithelium. This lung fluid maintains lung volume at about the functional residual capacity (FRC) and is a determinant of normal lung growth. A constant flow of this fluid is secreted into the alveolar spaces throughout development, which contributes to the fetal amniotic fluid.
Pulmonary and bronchial circulations also develop as the alveoli appear. Because of the compressive effect of the fetal lung fluid and the low alveolar partial pressure of oxygen (PA O2) in utero, the pulmonary capillary bed and pulmonary blood vessels remain constricted. High pulmonary vascular resistance and low pulmonary blood flow result.
The placenta provides the respiratory function for the fetus. The placental circulation has 2 major characteristics that enable the placenta to maintain adequate oxygenation of the fetus. First, the placenta has a multivillous circulation that provides the maximum surface area for the exchange of oxygen and carbon dioxide between the mother and fetus. Second, several factors result in the lowering of maternal pH and increasing of fetal pH, which results in increased transfer of oxygen from maternal to fetal hemoglobin or red blood cells (RBCs).
Maternal blood, carrying oxygen on adult hemoglobin, releases oxygen to the fetal circulation and accepts both carbon dioxide and various byproducts of metabolism from the fetal circulation. These transfers decrease maternal placental blood pH and shift the maternal oxygen-dissociation curve to the right, which results in lower affinity of the hemoglobin for oxygen and the release of additional oxygen to the fetal hemoglobin. The corresponding shift in the fetal oxygen-dissociation curve to the left allows the fetal hemoglobin to bind more oxygen. Additionally, the fetal circulation contains a higher hematocrit and hemoglobin content as well as fetal hemoglobin (HgbF), which has a higher affinity for oxygen. This higher affinity for oxygen allows the fetus to extract adequate oxygen in the maternal circulation at maternal physiologic saturations to allow for adequate fetal oxygen delivery to the tissues.
Fetal "breathing" (ie, chest wall and diaphragmatic movement) begins at approximately 11 weeks' gestation and increases in strength and frequency throughout gestation. Fetal breathing is controlled by chemoreceptors located in the aorta and at the bifurcation of the common carotid artery. These areas sense both pH and partial pressure of carbon dioxide (PCO2).
A reflex response to altered pH and PCO2 is present at approximately 18 weeks' gestation; however, the fetus is not able to regulate this response until approximately 24 weeks' gestation. Some studies have indicated that this response cannot be elicited in utero even when the pH and PCO2 are altered, leading researchers to believe that the response is suppressed in utero and is not activated until birth.
Studies also suggest that the low PA O2 in utero may be the mechanism that inhibits continuous breathing, finding that when PA O2 is increased, continuous breathing is stimulated. [2]
Neonatal pulmonary physiology
As noted (see above), the fetal airways and alveoli are filled with lung fluid that needs to be removed before respiration. Only a small portion of this fetal lung fluid is physically removed during delivery. During the thoracic squeeze, 25-33% of the fluid (sometimes markedly less) may be expressed from the oropharynx and upper airways. Thoracic recoil allows passive inspiration of air into the larger bronchioles. Effective transition requires that any remaining liquid be quickly absorbed by the lung tissues to allow effective gas exchange.
The decrease in lung fluid begins during labor. Studies using a fetal lamb model showed that the production of lung fluid decreases with the onset of labor. The subsequent reduction in lung fluid was associated with improved gas exchange and acid-base balance. Labor is also associated with increased catecholamine levels, which stimulate lymphatic drainage of the lung fluid.
With the onset of labor, the fetus produces adrenaline and the mother produces thyrotropin-releasing hormone, which stimulates the pulmonary epithelial cells to begin the adsorption of fluid. These findings could account for the increased incidence of transient tachypnea of the newborn after birth by cesarean delivery without antecedent labor.
After birth, lung fluid is removed by several mechanisms, including evaporation, active ion transport, passive movement from Starling forces, and lymphatic drainage. Active sodium transport by energy-requiring sodium transporters, located at the basilar layer of the pulmonary epithelial cells, drives liquid from the lung lumen into the pulmonary interstitium, where it is absorbed by the pulmonary circulation and lymphatics.
Exposure to an air interface, along with high concentrations of glucocorticoids and cyclic nucleotides, reverses the direction of ion and water movement in the alveoli and leads to highly selective sodium channels. These changes shift the fetal lung epithelial cells from a pattern of chloride secretion to one of sodium reabsorption, which accelerates reabsorption of fetal lung fluid.
The first breath must overcome the viscosity of the lung fluid and the intra-alveolar surface tension. This first breath must also generate high transpulmonary pressure, which helps drive the alveolar fluid across the alveolar epithelium. With subsequent lung aeration, the intraparenchymal structures stretch and gases enter the alveoli, resulting in increased PA O2 and pH. The increased PA O2 and pH result in pulmonary vasodilation and constriction of the ductus arteriosus.
Lung expansion and aeration is also a stimulus for surfactant release, which results in the establishment of an air-fluid interface and the development of Functional Residual Capacity (FRC). Normally, 80-90% of FRC is established within the first hour of birth in term neonates with spontaneous respirations. Premature and critically ill infants with surfactant deficiency or dysfunction may also have a limited ability to clear lung fluid and, consequently, an inability to establish a functional residual capacity (FRC) in the lungs.
The pulmonary vasculature is stimulated to dilate by chemical mediators, nitric oxide (NO), and prostaglandins. NO is released when pulmonary blood flow and oxygenation increases. The formation of certain prostaglandins, such as prostacyclin, is induced by the presence of increased oxygen tension. Prostacyclin acts on the pulmonary vascular smooth muscle bed to induce pulmonary vasodilation. It has a short half-life in the bloodstream and therefore does not affect the systemic circulation.
Soon after birth, fetal respiratory activity must transition to normal spontaneous breathing. To overcome the viscosity of the lung fluid and the resistance generated by the fluid-filled lungs and the recoil and resistance of the chest wall, lungs, and airways, the infant must generate a significant initial negative pressure so that air moves from the areas of higher pressure to lower pressure. There are 2 major physiologic responses that govern the initial lung inflation in the neonate.
The first response is the so-called rejection response, in which the active, non-depressed neonate responds to positive-pressure lung inflation by generating a positive intraesophageal pressure to resist the inflation. That is, the infant actively resists attempts to inflate the lungs by performing an active exhalation. This response not only acts to reduce lung inflation but also may cause high transient inflation pressures.
The second response is Head's paradoxical response, in which the neonate responds to positive-pressure lung inflation with an inspiratory effort, which generates a negative intraesophageal pressure. This inspiratory effort and the resultant negative pressure produce a fall in inflation pressures but result in a transient increase in tidal volume.
Of course, the depressed neonate may demonstrate no response to the inflation attempt and may not generate any change in intraesophageal pressure during positive-pressure inflation, in which case passive inflation results. These physiologic responses to positive-pressure inflation in the delivery room may cause large variability in tidal volume and intrapulmonary pressures, despite constant delivery of inflation pressure.
Stimuli for the first breath may be multifactorial. The environmental changes that occur with birth (eg, tactile and thermal changes and increased noise and light) activate a number of sensory receptors that may help initiate and maintain breathing. Clamping of the cord removes the low resistance placenta, causing an increase in systemic vascular resistance and consequently causing an increase in both systemic blood pressure and pulmonary blood flow.
Certain evidence also suggests that the increased arterial partial pressure of oxygen (Pa O2) after the initial breaths may be responsible for the development of continuous breathing via hormonal or chemical mediators that are still undefined.
When the newborn lungs fill with air, the Pa O2 should rise gradually. In term infants with a persistent hypoxia, an initial increase in ventilation effort occurs, followed by a decrease in ventilation efforts. This effect is even more profound in premature infants whose central nervous system (CNS) is not as mature.
The carotid bodies and peripheral chemoreceptors located at the bifurcation of the common carotid artery are stimulated during hypoxia to increase minute ventilation. In asphyxiated infants who cannot increase minute ventilation (eg, because of extreme prematurity or sedation), profound bradycardia may result.
Cardiovascular adaptation
Fetal circulation
To understand the cardiovascular changes that occur in the neonate at birth, an understanding of normal fetal circulation is necessary. The umbilical vein carries the oxygenated blood from the placenta to the fetus. Blood flow in this vein divides at the porta hepatis, with 50-60% of the blood passing directly to the inferior vena cava (IVC) via the ductus venosus and the remainder passing into the portal circulation. This portal blood flow perfuses the liver and then passes into the IVC.
Flow studies have revealed that relatively little mixing of the blood from these 2 sites occurs in the IVC. The more highly oxygenated blood, which has bypassed the liver, streams into the IVC to pass preferentially through the patent foramen ovale into the left atrium. The desaturated blood returning from the liver and lower body streams into the IVC to the right atrium.
In the right atrium, the desaturated blood mixes with blood returning from the coronary sinus and superior vena cava (SVC) and flows into the right ventricle. The more highly oxygenated blood that crosses the foramen ovale mixes with the small amount of pulmonary venous return and then crosses the mitral valve into the left ventricle. The output from the left ventricle passes into the ascending aorta to the heart, brain, head, and upper torso. The less saturated blood from the right ventricle passes into the pulmonary arteries.
Because the pulmonary vessels are constricted and highly resistant to flow, only about 12% of this blood from the right ventricle enters the lungs; the remainder takes the path of least resistance through the patent ductus arteriosus into the descending aorta. Approximately one third of this blood is carried to the trunk, abdomen, and lower extremities, with the remainder entering the umbilical artery, where it is returned to the placenta for reoxygenation.
Neonatal circulation
The aeration of the lung results in an increase in arterial oxygenation and pH, with a resulting dilation of the pulmonary vessels. Decompression of the capillary lung bed further decreases the pulmonary vascular resistance. A corresponding decrease in right ventricular and pulmonary artery pressures is also noted. The decrease in pulmonary vascular resistance leads to an increase in blood flow to the lungs and in pulmonary venous return.
Clamping of the umbilical cord removes the low-resistance placental vascular circuit and thereby raises total systemic vascular resistance, with a resultant increase in left ventricular and aortic pressures. The increased systemic vascular resistance, combined with the decreased pulmonary vascular resistance, reverses the shunt through the ductus arteriosus (from "fetal" right-to-left shunting to "neonatal" left-to-right shunting) until the ductus closes completely.
All of these events result in closure of the other fetal shunts. With the decrease in right atrial pressure and the increase in left atrial pressure, the 1-way "flap-valve" foramen ovale is pushed closed against the atrial septum. This functional closure at birth is followed by later anatomic closure, which usually occurs at several months of age.
The ductus venosus closes because of the clamping of the umbilical cord which terminates the umbilical venous return. Functional mechanical closure of the ductus venosus is accomplished by the collapse of the thin-walled vessels. Anatomic closure subsequently occurs at approximately 1-2 weeks.
Closure of the ductus arteriosus (a patent ductus arteriosus - PDA) is frequently delayed in preterm infants or infants with persistent pulmonary hypertension. The constriction and closure of the ductus arteriosus is accomplished by contractile tissue within the walls of this blood vessel. The contraction of this tissue is dependent both on the increase in arterial oxygen related to the onset of spontaneous respirations and on a fall in circulating prostaglandin E2 (PGE2).
Because the placenta is a major site of fetal PGE2 production, removal of the placenta from the circulation causes circulating fetal PGE2 concentration to decrease markedly. Further reductions in PGE2 concentration occur because of increased blood flow to the lungs (the site of PGE2 metabolism). Functional closure of the ductus arteriosus (PDA) in the normal term infant generally occurs within 72 hours of life, with anatomic closure by age 1-2 weeks.
In summary, functional postnatal circulation generally is established within 60 seconds; however, completion of the transformation can take up to 6 weeks.
Response to asphyxia
A fetus or newborn that is subjected to asphyxia (see the image below) initiates a "diving" reflex (so termed because of certain similarities to the physiology of diving seals) in an attempt to maintain perfusion and oxygen delivery to vital organs. Hypoxia and acidosis lead to pulmonary arteriolar vasoconstriction. Pulmonary vascular resistance increases, leading to decreased pulmonary blood flow and increased blood flow directly to the left atrium.
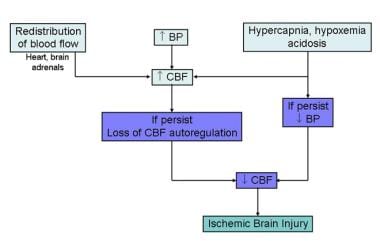 Fetal response to asphyxia illustrating initial redistribution of blood flow to vital organs. With prolonged asphyxial insult and failure of compensatory mechanisms, cerebral blood flow falls, leading to ischemic brain injury.
Fetal response to asphyxia illustrating initial redistribution of blood flow to vital organs. With prolonged asphyxial insult and failure of compensatory mechanisms, cerebral blood flow falls, leading to ischemic brain injury.
Systemic cardiac output is redistributed, with increased flow to the heart, brain, and adrenal glands and decreased flow to the rest of the body. Early in the course of asphyxia, systemic blood pressure increases. With ongoing hypoxia and acidosis, however, the myocardium fails and bradycardia occurs, resulting in a decrease in blood pressure and tissue perfusion, leading to eventual tissue ischemia and hypoxia.
Infants or fetuses who are undergoing asphyxia exhibit an altered respiratory pattern. Initially, they have rapid, frantic respiratory efforts. These respiratory efforts slow and then eventually cease with continued asphyxia (primary apnea). During primary apnea, the infant will respond to stimulation by re-initiation of breathing. However, if the asphyxia continues, the infant then begins irregular gasping respiratory efforts which then slowly decrease in frequency and eventually cease (secondary apnea).
Infants who experience secondary apnea do not respond to tactile or noxious stimulation and require positive-pressure ventilation (PPV) to restore ventilation. Primary and secondary apnea cannot be clinically distinguished. Therefore, if an infant does not readily respond to stimulation, PPV should be instituted as outlined in the Neonatal Resuscitation Program (NRP) guidelines.
If an infant is experiencing primary apnea, the stimulation of the ventilatory efforts causes the infant to resume breathing. If the infant is in secondary apnea, PPV is required for a longer period. The longer the infant undergoes asphyxia, the longer the onset of spontaneous respirations is delayed after the initiation of effective ventilation through the use of PPV.
Preparation for Resuscitation
Numerous sources of information are available on the training, skills, and procedures needed for delivery room resuscitation of the newborn. Among the most highly respected of these sources is the Neonatal Resuscitation Program (NRP), jointly developed by the American Academy of Pediatrics (AAP) and the American Heart Association (AHA). The following sections contain a review of resuscitation procedures in a format that is similar to the format used by the NRP.
Completion of the NRP training program should be considered by all hospital personnel who may be involved in the stabilization and resuscitation of neonates in the delivery room. To develop true expertise, additional supervised experience with skilled personnel is essential.
Although the NRP is considered highly authoritative, it is important that more research continue to evaluate the effectiveness of the techniques of neonatal resuscitation. The NRP has already evolved considerably and will continue to evolve as new data from clinical studies and basic physiologic research become available.
Rapid assessment
Newborn infants who need extensive resuscitation should be rapidly identified. Term infants with clear amniotic fluid, adequate respiratory effort, and good muscle tone should receive routine care, which includes provision of warmth, clearing of the airway (if needed), drying of the infant, and assessment of the infant's color. These infants should remain with their mothers during and after routine care.
Infants who do not meet the criteria for routine care need additional steps in their resuscitation. For such infants, resuscitation may include not only initial stabilization (providing warmth, positioning, clearing the airway, drying, stimulating, and repositioning) but also ventilation, chest compressions, and medications.
Anticipation of potential problems
The goals of resuscitation are to assist with the initiation and maintenance of adequate ventilation and oxygenation, adequate cardiac output and tissue perfusion, and normal core temperature and serum glucose. These goals may be attained more readily when risk factors are identified early, neonatal problems are anticipated, equipment is available, personnel are qualified and available, and a care plan is formulated.
A large number of antepartum and intrapartum maternal conditions carry an increased risk for intrapartum asphyxia. Many excellent texts describe the extensive medical and surgical problems of the obstetrical patient; a detailed review of these problems is beyond the scope of this article to review.
Resuscitation equipment
The delivery room should be equipped with all the tools necessary for successful resuscitation of a newborn of any size or gestational age. The equipment should include a radiant warmer, warmed blankets, a source of oxygen, instruments for visualizing and establishing an airway, a source of regulated suction, instruments and supplies for establishing intravenous (IV) access, trays equipped for emergency procedures, and drugs that may be useful in resuscitation.
Respiration equipment includes the following:
-
Stethoscope
-
Cardiorespiratory leads for neonates and cardiorespiratory monitor
-
Pulse oximeter leads (neonatal size) and pulse oximeter monitor
-
Oxygen supply with blender, set to 10 L/minute
-
Assorted masks (term and preterm mask sizes)
-
Positive Pressure Ventilation (PPV) device and tubing connected to the blended oxygen source
-
Manometer (or appropriately calibrated T-piece PPV device)
-
Endotracheal tubes (sizes 2.5, 3.0, 3.5)
-
Tape and scissors or other devices for anchoring endotracheal tubes
-
Laryngoscope (Size 0 and 1 straight blades, Size 00 optional)
-
Extra bulbs and batteries
-
Carbon dioxide detectors
-
Stylettes for endotracheal tubes (optional)
-
Laryngeal mask airway, Size 1 (optional)
Suction equipment includes the following:
-
Bulb syringe
-
Regulated mechanical suction (80-100 mm Hg)
-
Suction catheters (10F and/or 12 F)
-
Suction tubing
-
Suction canister
-
Replogle or Salem sump vented suction tubes (10 F - 12F)
-
Feeding tube (8 French catheter)
-
Syringe, catheter-tipped (20 mL)
-
Meconium aspirator
Fluid equipment includes the following:
-
IV catheters (22 g, 24 g)
-
Tape and sterile dressing material
-
Dextrose 10% in water (D10W)
-
Normal Saline solution
-
T-connectors
-
Syringes, assorted (1-20 mL)
Drugs:
-
Epinephrine (1:10,000 - 0.1 mg/ml)
-
Note: This should be the ONLY epinephrine concentration available
-
Normal Saline
Procedural equipment includes the following:
-
Umbilical catheters (2.5 F and 5 F)
-
Chest tube (10 French catheter)
-
Sterile procedure trays (eg, scalpels, hemostats, forceps)
Trained personnel
For all deliveries, at least 1 person should be present who is skilled in neonatal resuscitation and is responsible only for the infant. This person must be skilled in the initiation of resuscitation, the set-up and use of positive pressure ventilation, and the performance of chest compressions.
Additional personnel should be immediately available to assist in tasks that may be required as part of resuscitation, including intubation, medication administration, and emergency procedures, if needed. If the delivery is identified as high-risk, at least 2 or more skilled individuals should be assigned to the infant at delivery.
Remember that staff trained in neonatal resuscitation must apprentice with experienced personnel for some time before they can be independently responsible for an infant at a delivery. Simulation training has become an increasingly important component of training in neonatal resuscitation. [3]
Resuscitation of Neonates
Thermoregulation
Preventing heat loss during resuscitation is essential. Intrauterine thermoregulation is passive, with no use of calories or oxygen by the fetus. This passive thermoregulation process allows the fetus to achieve maximal intrauterine growth without having to expend energy on thermal homeostasis. Brown fat storage begins during the third trimester. Brown fat may be used for heat production in the newborn period.
Several factors lead to increased heat losses in the newborn. Neonates have a high ratio of skin surface area to body weight, which increases transdermal heat loss and evaporative fluid loss. The fluid loss from the skin (due not to sweating but to direct transdermal water loss) results in massive evaporative heat loss. The thin fetal skin, with blood vessels that are near the surface, provides poor insulation, which leads to further heat loss.
Additionally, the newborn infant (especially if premature) has a limited capacity to change body position for heat conservation. Animals ordinarily attempt to decrease heat loss by decreasing their exposed surface area (eg, by curling up). This reduction in exposed surface area is accomplished by assuming a flexed position; however, premature, critically ill, and depressed infants are unable to accomplish flexed positioning.
Neonates have a very limited capacity for metabolic heat production. The newborn infant has limited energy stores, largely because of decreased subcutaneous fat and brown fat stores, and this paucity of fat stores is more pronounced in premature and growth-retarded infants. Moreover, infants are not capable of effective shivering, which is a major source of heat production in the adult. The main source of heat production in the newborn is nonshivering thermogenesis.
Thermoreceptors in the face are markedly sensitive to heat and cold. Cold stimulation leads to norepinephrine production and thyroid hormone release, causing brown fat to be metabolized. Brown fat is highly vascularized and stored in pockets around the neonate's body. When it is metabolized, triglycerides are hydrolyzed to fatty acids and glycerol. Additionally, glycolysis is initiated and glycogen stores are used, both of which result in glucose production. Heat is produced as a byproduct of the increased metabolic rate and oxygen consumption.
Infants who experience heat loss have an increased metabolic rate and use more oxygen. Increased oxygen consumption can be dangerous in infants who are experiencing respiratory compromise. The addition of cold stress in infants who are poorly oxygenated can potentially trigger a change from aerobic to anaerobic metabolism. This change in metabolism may lead to tissue hypoxia and acidosis because of the buildup of metabolic byproducts such as lactate.
Because of the inefficiency of anaerobic metabolism, the infant uses up glucose and glycogen reserves rapidly while still generating only a limited amount of energy for heat production. Therefore, cold stress can lead to both metabolic acidosis and hypoglycemia. Infants with asphyxia have thermoregulatory instability, and hypothermia delays recovery from acidosis.
Hypothermia on admission to the neonatal unit has been shown to be associated with an increased mortality. [4] In view of this finding, it is clearly essential to prevent excessive heat loss in the delivery room and throughout stabilization and transport to the neonatal unit. Normothermia and hypothermia in infants have been defined by the World Health Organization (WHO) as outlined in Table 2 below.
Table 2. Axillary Temperatures in Infants Weighing Less Than 1500 g (Open Table in a new window)
Ranges |
Temperature |
Action Needed |
Normal |
36.5-37.5o C |
Continue |
Potential cold stress |
36-36.5o C |
Cause for concern |
Moderate hypothermia |
32-36o C |
Danger; immediate CONTROLLED warming of baby needed |
Severe hypothermia |
< 32o C |
Outlook grave; skilled care urgently needed |
The American Heart Association (AHA) and the American Academy of Pediatrics (AAP) have stated that the goal (of the first temperature) should be an axillary temperature of 36.5o C. The aim is to achieve normothermia and avoid hyperthermia, which is associated with progressive cerebral injury.
The environmental temperature is also important in controlling heat loss in the newborn. For a fetus, the thermal environment is precisely regulated by the mother's core temperature, and heat losses are nonexistent. After delivery, even with drying and the use of a radiant heat source, infants continue to lose large amounts of heat through convective and evaporative mechanisms. When the environmental air is cooler than the neutral thermal environment for the infant being resuscitated, further thermal losses ensue.
Heat losses are related both to the absolute temperature gradient and the difference between the skin and the air water concentration. The primary goal in neonatal thermoregulation is prevention of heat loss, as opposed to later correction of heat loss through rewarming. Ideally, a specific area (eg, a stabilization room) should be maintained separate from the operating room (OR) or labor room so that special attention can be paid to the unusual thermal and environmental needs of the newborn high-risk infant.
This stabilization area should be kept as warm as possible, with the requirements of the high-risk infant balanced against the comfort of the adult staff in that area. Low delivery room temperatures can predispose to hypothermia, and Neonatal Resuscitation program (NRP) guidelines recommend that if a preterm delivery is anticipated, the delivery room temperature should be increased. Ideally, a dedicated room would be available in which ambient temperature can be well controlled.
Suggested delivery room temperatures by age and weight (determined on the basis of consensus groups—still considered an evolving clinical practice) are as follows [5] :
-
Estimated gestational age (EGA) less than 26 weeks, estimated birth weight (EBW) less than 750 g, or both - 76o F or higher, target 78-80o F
-
EGA 27-28 weeks, EBW less than 1000 g, or both - 74o F or higher, target 78-80o F
-
EGA 29-32 weeks, EBW 1001-1500 g, or both - 72o F, target 75o F
-
EGA 33-36 weeks, EBW 1501-2500 g, or both - 72o F, target 75o F
-
EGA 27-42 weeks, EBW greater than 2500 g, or both - 70o F, target 75o F
Newborns should be dried with prewarmed blankets or towels and placed on a prewarmed heat source with an insulated mattress. Open bed warmers, which use radiant heat, are commonly used in delivery rooms. They provide warmth during resuscitation and for any subsequent invasive procedures. However, it is important for the practitioner to keep in mind that this source of heat does not protect the infant from evaporative heat loss but, instead, encourages evaporative heat losses.
Continuous monitoring of temperature should occur as soon as possible after the delivery. Premature infants (< 1500 g) should be covered in plastic wrap (polyethylene) to prevent excessive heat loss. A full resuscitation, including line placement, can and should be performed with the plastic wrap in place. A woolen head cap should also be used.
Weights should be obtained on radiant warmer bed scales. Adequately pre-warming the transport incubator is essential. The infant's temperature should be documented as soon as possible after birth and every 10-15 minutes thereafter until continuous temperature monitoring has been established.
Another common source of heat loss in the neonate undergoing resuscitation is the use of unheated, nonhumidified oxygen sources for the PPV devices. The inspired gases sent to the lungs are subsequently heated and humidified by the infant; this results in massive heat exchange from evaporative heat loss and insensible water loss. Whenever possible, warmed and humidified gases should be provided in the resuscitation area. Alternatively, the intubated and ventilated infant should be placed on a heated ventilator circuit as soon as is feasible.
Airway management
Once in a heated environment, the infant should be positioned so as to open the airway, and the mouth and nose should be suctioned. A bulb syringe may be used for the initial suctioning, taking care not to evoke a bradycardic response with vigorous suctioning.
Infants have a vagal reflex response to sensory stimulation of the larynx, which may induce apnea, bradycardia, hypotension, and laryngospasm. Thus, suctioning the posterior oral airway or the trachea with a catheter because of extremely thick or meconium-stained fluids may cause profound central apnea, and potentially eliciting profound bradycardia and laryngospasm. Accordingly, it should be limited to infants with thick mucus that cannot be removed by bulb syringe or used for the aspiration of stomach contents (when necessary).
Instillation of saline into the trachea also has been shown to stimulate the afferent sensory neurons leading to these adverse sequelae and consequently has no place in the immediate resuscitation period. Lung inflation has been shown to reverse the effects of vagal stimulation. Vigorous suctioning of the nares with a catheter can lead to edema of the nasal tissues with resulting respiratory distress after the infant leaves the delivery room. Wall suction should be set so that pressures do not exceed 100 mm Hg.
Stimulation
Drying and suctioning often provide enough stimulation to initiate breathing; however, if more vigorous stimulation is necessary, slapping the soles of the feet or rubbing the back may be effective. The back should be visualized briefly for any obvious defect in the spine before beginning these maneuvers.
If there is no response to stimulation, it may be assumed that the infant is in secondary apnea, and positive-pressure ventilation (PPV) should be initiated. At this point, the infant's respiratory rate, heart rate, and color should be evaluated. Most infants do not require further intervention. This is considered routine care for most term infants with clear amniotic fluid who are actively breathing and crying and have good muscle tone.
Supplemental oxygen
Infants who do not meet the criteria for routine care or who have difficulties with respiratory effort, tone, or color need further intervention. Further resuscitative efforts should be guided by simultaneous assessment of respirations, heart rate, and color.
Most infants need observational care. Neonatal transition occurs over time. Infants who have a sustained heart rate higher than 100 beats/min and adequate respiratory effort but who remain cyanotic should receive blow-by oxygen via oxygen tubing or a mask, guided by a pre-ductal saturation probe reading. Heated humidified oxygen is arguably advantageous, but it is rarely available in the delivery room environment.
Supplemental oxygen should be initially provided as required by the measured pre-ductal saturation values of the infant. While infants were previously administered 100% oxygen for all resuscitation interventions, it has been determined that resuscitation with room air (21% oxygen) is at least as effective as resuscitation with 100% oxygen. Oxygen administration should be guided by attaining the appropriate pre-ductal saturation target as referenced in the NRP Program. If supplemental oxygen is to be provided for a prolonged period, heated humidified oxygen should be supplied via an oxygen hood, with the FI O2 adjusted to result in pulse-oximetry saturations of 92-96% in the term infant and 88-92% in the preterm infant.
Term infants should be initially be resuscitated with a FI O2 of 21%, but this value should be increased if the infant does not improve with adequate ventilation. In premature infants, oxygen should be on a blender and blended up or down to keep the saturation around 90%.
Positive-pressure ventilation
For a number of reasons (see Transition to Extrauterine Physiology), it can be difficult for the infant to clear fluid from the airways and establish air-filled lungs. Initial respiratory efforts may have to be augmented by the addition of either continuous positive airway pressure (CPAP) or PPV.
Postresuscitative care is the term used for the management of infants who require more extensive resuscitation. The addition of positive pressure aids in the development of functional residual capacity (FRC) and is needed more commonly in premature infants. Mechanical lung inflation is also important to reverse persistent bradycardia in an apneic asphyxiated infant. Call for assistance when beginning PPV if other team members are not already in attendance.
Infants with adequate respirations who are experiencing respiratory distress (manifested by tachypnea, grunting, flaring, retracting, or persistent central cyanosis) may benefit from positive end-expiratory pressure (PEEP) by the application of CPAP. If the infant is apneic, is making inadequate respiratory efforts (gasping), or has a heart rate lower than 100 beats/min, Positive Pressure Ventilation (PPV) should be initiated immediately. Infants who have continued central cyanosis despite supplemental oxygen should also receive PPV.
The ideal PPV device is equipped to deliver PEEP via an appropriately sized mask applied firmly to the face. A T-piece resuscitator device provides a calibrated and constant PEEP and is capable of delivering a measured inflation pressure as required for PPV. It has been shown to be as effective as flow-inflating and self-inflating bags. It allows more precise delivery of inflation pressure and inspiratory times. [6]
In term infants, an FI O2 of 0.21 should be used when PPV is started. If the infant remains cyanotic, or below the expected saturation value for age, then the FI O2 should be increased as required. If supplemental oxygen is not available, CPAP or PPV utilizing room air should be used.
Premature infants (< 32 wk) who require PPV should begin with oxygen concentrations between room air and 30% and have the subsequent administration of oxygen guided by the pre-ductal pulse oximetry reading. If the oxyhemoglobin concentration rises to about 95%, oxygen should be weaned. Any infant who does not respond to PPV with a heart rate of about 100 beats/min should be placed on an FI O2 of 1 and have the mask repositioned or be intubated.
Some infants respond to brief mechanical ventilation and subsequently begin independent ventilation; others need continued ventilatory support. Sufficient, but not excessive, initial pressure must be used to adequately inflate the lungs, or else bradycardia and apnea will persist.
A pressure manometer should always be used with a pressure release valve, limiting the positive pressure to 30-40 cm H2O during the first breaths. This may have to be reduced to 20-24 cm H2O in preterm infants with an increase in pressures if the chest does not rise or the heart rate does not rapidly increase. [7] To provide adequate distending pressure, the infant must be properly positioned and the upper airway must be cleared of secretions; the mask must be the correct size and form a tight seal on the face.
When assisted breaths are being provided, the primary measure of adequate initial ventilation is a rapid increase in heart rate. Auscultation of breath sounds should be performed, but may be difficult to interpret in a tiny infant. A rise and fall in the chest wall movement is not always adequately assessed. [7] If no chest rise occurs, either the airway is blocked or insufficient pressure is being generated by the squeezing of the bag.
Ventilatory rates of 40-60 breaths/min should be provided initially, with proportionally fewer assisted breaths provided if the infant's spontaneous respiratory efforts increase. Although this practice has not been extensively studied, initial inflation of the newborn's lungs with either slow-rise or square-wave inflation to a pressure of 30-40 cm H2 O for approximately 5 seconds has been reported to result in more rapid formation of FRC.
At the moment of delivery and first breath, the neonatal lung is converting from a fetal nonaerated status to a neonatal status. The neonatal lung has a requirement for gas exchange, and this necessitates the development of FRC with the resorption of lung fluid and the resolution of most of the atelectasis. Therefore, initial slow ventilation with more prolonged inspiratory times may be useful to assist in this task, balanced against the need to avoid inappropriate inspiratory pressures.
Flow-controlled, pressure-limited mechanical devices are acceptable for delivering PPV. These mechanical devices control flow and limit pressure and have been shown to be more consistent than bags. Self-inflating and flow-inflating bags remain a standard of care. Laryngeal mask airways are effective for assisted ventilation when bag-mask ventilation and intubation are unsuccessful.
Premature infants are at high risk for lung injury from large-volume inflation (volutrauma). Carefully observing the chest expansion and monitoring the pressure used in these patients while providing consistent inflations without over-inflation is essential. Initial inflation pressures of 20-25 cm H2O are usually adequate. Higher pressures may be needed if no improvement in heart rate or chest movement is noted. CPAP is beneficial in premature infants once they are breathing spontaneously.
The effectiveness of assisted ventilation should be evaluated by observing an increase in heart rate. Other signs that should be monitored include improvement in color, spontaneous breathing, and improvement in muscle tone. All of these signs should be assessed within 30 seconds of PPV administration.
A study to investigate if postresuscitation care (PRC) is indicated for all infants who receive PPV at birth concludes that neonates who receive PPV at birth for as little as one minute still need close monitoring as part of their postresuscitation care. [8, 9]
Intubation
Infants may require tracheal intubation if direct tracheal suctioning is required, effective bag-mask ventilation cannot be provided, chest compressions are performed, endotracheal (ET) administration of medications is desired, congenital diaphragmatic hernia is suspected, or a prolonged need for assisted ventilation exists.
See the video on assisted ventilation in the newborn, below.
An appropriate blade (Miller size 0 or 1) should be chosen in accordance with the size of the infant. Premature infants may be more easily intubated with a size 0 blade, and term infants require a size 1 blade. An appropriately sized endotracheal tube should be chosen in accordance with the weight of the infant (see Table 3 below).
Table 3. Endotracheal Tube Size and Measurement at Lip According to Infant Weight (Open Table in a new window)
Infant Weight |
Endotracheal Tube Size |
Endotracheal Tube Measurement at Lip |
< 1000 g |
2.5 |
7 cm |
1000-2000 g |
2.5-3 |
8 cm |
2000-3000 g |
3-3.5 |
9 cm |
> 3000 g |
3.5 |
10 cm |
Once inserted, the ET tube should be advanced until the vocal cord guide mark near its distal tip is observed to be slightly past the vocal cords. This guide mark is positioned a variable distance from the distal tip (depending on the tube size) and is designed to result in the placement of the tube tip between the vocal cords and the carina at the bifurcation of the right and left mainstem bronchi. Once correctly positioned, the ET tube should be secured and cut to an appropriate length to minimize dead space and flow resistance.
Another way of estimating correct placement of the ET tube is to take the weight of the infant in kilograms and add 6 to yield at the number of centimeters at which the tube should be secured at the lip. Before the tube is secured, the infant should be assessed for equal bilateral breath sounds with maintenance of oxygenation. An increase in the heart rate within 5-15 seconds is an excellent indicator of adequate ventilation and appropriate ET tube placement.
Measurement of exhaled carbon dioxide provides secondary confirmation. Carbon dioxide detectors use a colorimetric change to indicate exhalation of the gas. The use of such detectors is the only technique that has been evaluated for confirmation of ET tube placement in infants and is therefore recommended. [10, 6] When carbon dioxide detectors are used in infants with poor pulmonary blood flow that cannot deliver sufficient carbon dioxide to the lungs, a false negative result may occur, leading to unnecessary extubation.
Ultimately, ET tube position is confirmed with chest radiography. Free-flow oxygen should be provided throughout the procedure, and effective ventilation should be provided via the PPV device or ventilator after the infant is intubated.
Cardiovascular support and chest compressions
Most infants who present at delivery with a heart rate lower than 100 beats/min respond to effective ventilatory assistance by rapidly increasing their heart rate to normal levels. In contrast, if an effective airway and effective ventilation are not established, further support is not effective. Chest compressions should be initiated if the heart rate remains below 60 beats/min and only if effective PPV is being accomplished via a secured airway (intubated). Proceeding to chest compressions and/or drug therapy will not be effective without effective airway management and respirations.
In an editor's note commenting on an article addressing cardiopulmonary resuscitation in the delivery room, [11] Catherine DeAngelis wrote, "[C]heck the airway (optimize respiratory support) one more time before compressing the chest. More often than not, you and the infant can then take deep breaths, and you can beat your own chest instead of the infant's."
An assessment of the heart rate can be obtained through direct auscultation of the precordium. Chest compressions should be discontinued as soon as the heart rate is 60 beats/min or more.
Chest compressions should be performed by circling the chest with both hands and using the thumbs to compress the sternum. This thumb technique is recommended because it allows better depth control during compressions. To be effective, the chest should be compressed approximately one-third of the anterior-posterior (AP) diameter of the chest. This technique is capable of generating adequate peak systolic pressures and coronary perfusion. Chest compressions from the head of the bed may be required for umbilical line placement and medication administration.
Pressure should be applied to the lower portion of the sternum, depressing it to a depth of about one third of the anterior-posterior diameter. The chest should fully reexpand during relaxation. One ventilation should be interposed after every 3 chest compressions. An overall rate of 120 compression/ventilation events per minute is recommended; with the 3:1 compression-to-ventilation ratio, this equates to 90 compressions and 30 breaths each minute.
Evaluate heart rate and color every 60 seconds. Infants who fail to respond may not be receiving effective ventilatory support; thus, constantly evaluating ventilation is imperative. Chest compressions should be discontinued when the heart rate is 60 beats/min or higher.
Medications
Neonatal resuscitation drugs should be stocked in any area where neonates are resuscitated, including each delivery and stabilization area, as well as the emergency department (ED). Personnel should be familiar with neonatal medications, concentrations, dosages, and routes of administration. Drugs currently recommended include epinephrine (1:10,000) and isotonic sodium chloride solution (0.9%) as an intravascular volume expansion agent.
Epinephrine should be considered only when the heart rate is below 60 beats/min and effective ventilation (usually with an intubated infant) has been established and provided for at least 30 seconds, followed by a full 60 seconds of compressions and effective ventilation. The recommended dose is 0.01-0.03 mg/kg (0.1-0.3 mL of the 1:10,000 solution), preferably administered intravenously (IV). Higher IV doses are not recommended, and the post-resuscitation hypertension could put premature infants at risk for intraventricular hemorrhage.
If vascular access cannot be obtained, epinephrine may be given via the ET tube, but in such cases, the dose should be increased to 3 times the IV dose. To ensure that the small volume is not deposited on the ET tube connector or in the lumen of the tube, administration of epinephrine should be followed by 5 quick breaths to ensure that the drug is delivered to the lung, where it is should be absorbed and delivered to the heart.
If an umbilical venous catheter is used for medication administration, the catheter should be inserted only as far as the point where blood flow is obtained (usually 2-4 cm). Because the dosing recommendations for epinephrine include ET administration, the need for emergent placement of umbilical venous catheters has been reduced markedly in the delivery room.
In the study described in this article, approximately one third of the infants with neonatal depression at birth had associated fetal acidemia. [11] However, in the remaining infants without fetal acidemia, chest compressions were initiated as a consequence of improper or inadequate ventilatory support at birth. This explains why the NRP recommendations have been changed to emphasize airway management and effective ventilation.
In the population of infants without initial acidemia, chest compression or epinephrine therapy was ineffective. [11] The heart rate only improved after effective tracheal intubation established a patent airway or after incremental increases in PPV exceeded the opening pressure of the lungs, establishing ventilation.
This study and others continue to reinforce the primary importance of the establishment of effective ventilation. Without ventilation, other therapies, including medications, will not be effective in establishing adequate heart rate and perfusion.
Sodium bicarbonate had previously been recommended in the delivery room to reverse the effects of metabolic acidosis related to hypoxia and asphyxia. However, studies show that 0.9% saline provides better cardiac and blood pressure support to correct both the metabolic acidosis itself and the underlying cause of the acidosis. Use of sodium bicarbonate in the delivery room has been associated with an increased incidence of intraventricular hemorrhage in very low birth weight infants.
Volume expansion may be used in neonates with evidence of acute blood loss or with evidence of shock of any etiology. In general, the neonatal heart responds well to the increase in preload at the atrial level caused by the volume expansion. Hypovolemia may be masked in a newborn infant because of the significant peripheral vasoconstriction caused by the elevation in catecholamine levels after delivery. Systolic blood pressure also may be elevated due to with pain.
Currently, isotonic sodium chloride solution (Normal Saline) is the only recommended volume expansion during resuscitation. The recommended dosage of isotonic sodium chloride solution for volume expansion is 10 mL/kg IV over 5-10 minutes; the solution may be infused more cautiously in extremely preterm infants. When blood loss is known, one may consider the use of O-negative packed red blood cells (RBCs). Restoring the critical oxygen-carrying capacity is essential.
Immediate Postresuscitation Period
Maintenance of airway and ventilation
The goal of delivery room management is to stabilize the airway and ensure effective oxygenation and ventilation.
Once initial lung recruitment is obtained, avoiding overdistention is essential. Breaths delivered by bag-mask ventilation may be difficult to control and may result in overdistention and consequent pneumothorax or pneumomediastinum. Additionally, the unheated nonhumidified oxygen can quickly cool the infant via the large surface area of the lungs, resulting in hypothermia. Therefore, mechanical ventilation should be initiated as soon as possible once the infant is stabilized.
Although the ideal mode of assisted ventilation is controversial, providing adequate positive end-expiratory pressure (PEEP) to prevent atelectasis, while at the same time preventing overinflation, is indicated. Once the appropriate functional residual capacity (FRC) is obtained, it is essential to use the lowest possible level of support that still allows adequate oxygenation and ventilation. Newer mechanical ventilators which allow sensing and quantitation of the neonatal ventilation and adjustment of the delivered ventilation are becoming more common-place in the NICU.
Oxygen saturation should be monitored continually and arterial blood gas analysis performed as needed during the initial stabilization period. Saturations should be maintained in the 90-96% range for the term infant and in the 90-92% range for the preterm infant after the initial stabilization.
Fluid and electrolyte management
In utero, nutrients are provided in their basic form. Glucose is the major energy substrate of the fetus. Fetal glucose uptake parallels maternal blood glucose concentration. The liver, heart, and brain receive the greatest cardiac output and consequently the largest amount of glucose. The fetus uses glucose, lactate, and amino acids to store fuels that are used during transition.
Neonates must develop a homeostatic balance between energy requirements and the supply of substrate as they move from the constant glucose supply of fetal life to the normal intermittent supply of glucose and other fuels that is characteristic of extrauterine life. With the clamping of the cord, the maternal glucose supply is immediately terminated. A fall in blood glucose during the first 2-6 hours of life occurs in term, healthy newborns. The blood glucose usually reaches a nadir and stabilizes at 50-60 mg/dL.
The immediate goals of fluid and electrolyte support after resuscitation are to maintain an appropriate intravascular volume and to achieve glucose homeostasis and electrolyte balance. The neonatal cardiovascular system is very sensitive to preload, requiring adequate intravascular volume to maintain adequate cardiac output. Therefore, expansion of intravascular volume with appropriate solutions (eg, isotonic sodium chloride solution) often is considered in the neonate with inadequate blood pressure or markedly reduced perfusion.
Additionally, hypoglycemia may occur rapidly in critically ill or premature infants. Blood glucose determinations should be performed as soon as possible, and a continuous infusion of glucose should be started at 4-6 mg/kg/min for infants who are unable to tolerate enteral feedings.
Dextrose boluses should be limited to symptomatic hypoglycemic infants because they may result in transient hyperosmolarity and rebound hypoglycemia. Dextrose bolus dosing should be (only) 2 ml/kg of D10W, usually followed by the administration D10 IV fluids for maintenance. Electrolytes (eg, sodium, potassium, and chloride) should not be added initially, because the fluid shifts from other body compartments allow adequate electrolyte supply until adequate renal function is documented. The volume of dextrose fluid bolus should not be confused with the 10-20 ml/kg of saline for intravascular volume replacement.
The practitioner should monitor the weight, clinical hydration status, urine output, and serum sodium concentrations closely because inappropriate fluid overload or restriction can lead to increased mortality and morbidity. The infant's environment must be taken into account in the calculation of fluid requirements. Fluid may be started at a rate of 60-80 mL/kg/day for the infant who is immediately placed in a humidified incubator, whereas it may have to be given at a much higher rate for the infant in a dry radiant-warmer environment.
Preparation for transport
Preparation of the infant for transfer to a remote nursery for care after resuscitation involves the following key considerations (see Transport of the Critically Ill Newborn):
-
Complete all the routine care that is required of newborn infants; these basics of care may be neglected in the rush to prepare the infant for transport, with potentially disastrous results
-
Secure all lines, tubes, catheters, and leads for transport; monitoring in the transport environment is possible only with functioning leads in place, which is frequently difficult to accomplish
-
Provide rapid and complete documentation of the resuscitation and subsequent therapies for the use of future caretakers
Special Problems During Resuscitation
A number of congenital and other neonatal conditions may present in the delivery room and may have an effect on the course of resuscitation. The most important of these are briefly reviewed below.
Extreme prematurity
Premature infants have special needs that must be considered during the critical period immediately after delivery if mortality and morbidity are to be decreased in this group. This population is at increased risk for respiratory failure, insensible water losses, hypoglycemia, and intraventricular hemorrhage. A full discussion of the many difficulties of extreme prematurity is beyond the scope of this article, but additional information may be found elsewhere (see Prematurity).
Insensible water loss in the premature infant is increased secondary to the infant's poorly cornified epidermis and immature stratum corneum, which presents only an insignificant barrier to evaporative heat loss. The stratum corneum is not functionally mature until 32-34 weeks' gestation. Differences in skin maturity, prenatal nutritional status, ventilation requirements, and environmental conditions all may influence the magnitude of insensible water loss that occurs after birth.
The skin is the most important route for water depletion after delivery of the extremely immature infant. Transepidermal water loss (TEWL) is highest at birth in infants who are born before 28 weeks' gestation and decreases slowly with advancing gestational age. Despite declines in TEWL with advancing age, infants born before 28 weeks' gestation continue to have increased TEWL for 4-5 weeks after birth, compared with infants born at term.
Because of high evaporative loss with the associated heat loss, the ability to achieve and maintain thermoregulation is compromised further. The skin barrier dysfunction increases the risk of infection, especially with organisms that colonize the skin surface (eg, staphylococcal species). This thin skin barrier also places the extremely immature infant at risk for toxic reactions to topically applied substances. Additionally, skin integrity is easily disrupted by the use of adhesives, which should be limited in premature infants.
Premature infants need increased fluid administration rates initially if they are on radiant warmers for a prolonged period. With increased parenteral fluid administration using dextrose-containing fluids, the dextrose must be monitored closely to ensure euglycemia. Placing infants in a humidified environment with patient servo-control decreases TEWL, improves maintenance of body temperature, and does not delay skin maturation.
Measures to decrease insensible water loss should be initiated at delivery. Because radiant warmers are often used routinely at deliveries out of a need for maximal patient access, infants weighing less than 1000 g should be wrapped in a plastic blanket or other barrier to decrease evaporative water loss until they can be placed in a humidified environment. However, care should be taken to ensure that the barrier does not block the transmission of heat from the radiant source.
Premature infants are at risk for intraventricular hemorrhage and periventricular leukomalacia (PVL) secondary to their immature cerebral vascular regulation and the persistence of the germinal matrix. These disorders are often associated with subsequent serious permanent neurodevelopmental disabilities.
Prevention of these pathological conditions or reduction of their severity may begin in the delivery room. Mechanical ventilation and fluid administration must be managed cautiously in this group of infants. Volume expansion should be administered only in the face of true hypotension. Knowledge of normal blood pressure values for infants of various gestational ages is essential. Volume expansion in the face of normal blood pressure increases the risk of intraventricular hemorrhage.
Additionally, it is important to generally avoid administration of any hyperosmolar medications (eg, sodium bicarbonate). Mechanical ventilation may lead to harmful fluctuations in cerebral blood flow, especially when the partial pressure of carbon dioxide (pCO2) and pH are rapidly altered. Rapid alterations in pCO2 and pH result in acute fluctuations in the cerebral blood flow of the premature infant with immature cerebral vascular autoregulation.
Premature infants are also at high risk for volutrauma caused by poor lung compliance and overventilation. Following the administration of exogenous surfactants with a subsequent rapid improvement of pulmonary compliance, overexpansion and volutrauma is possible unless those rapid changes in lung compliance are not monitored carefully. Overventilation with excessive tidal volumes and hypocarbia is associated with subsequent chronic lung disease.
Stabilization of the infant using the lowest peak inspiratory pressure (PIP) that will still yield adequate oxygenation and ventilation is essential. Hand ventilation of an intubated infant, especially when done by inexperienced personnel, will lead to inconsistent tidal volumes and pressures. Use of a mechanical ventilator designed for infants offers the advantages of more consistent tidal volumes and a reduction of the heat losses associated with the use of unheated nonhumidified air in hand ventilation. With improvements in ventilator technology, monitoring of the delivered pressures, volumes and minute ventilation are now possible. It remains to be seen if volume ventilation in the neonatal population will prove superior and result in less pulmonary trauma.
Although artificial surfactant administration is associated with a reduction of adverse sequelae in infants, it may lead to hyperventilation and overdistention if not carried out by experienced and attentive personnel. After the instillation of artificial surfactant, personnel must remain alert so that they can react rapidly to changes in pulmonary compliance to prevent the onset of hypocarbia and alkalosis.
After the institution of mechanical ventilation, care should be taken with airway suctioning because vigorous or frequent airway suctioning is associated with hypoxia, intraventricular hemorrhage (IVH), and periventricular leukomalacia (PVL). Prematurity with respiratory distress syndrome (RDS) is not associated with mucus production in the first 24 hours of life; thus, suctioning protocols should be altered to provide minimal deep pulmonary suctioning during this time.
Airway problems
Choanal atresia
Choanal atresia is caused by a failure of embryologic regression of nasal airway tissue, which results in partial or complete occlusion of the nasal airway. These choanal defects may be bony or membranous, with most having a bony component. Complete bilateral stenosis usually results in a neonatal respiratory emergency at birth because infants generally are obligate nasal breathers during the first 6-8 weeks of life. At rest, these infants usually manifest severe apnea, retractions, and respiratory distress that may be relieved with crying.
Wheezing or stridor may be audible with inspiration, and collapse of the small airways with vigorous inspiratory effort can occur. The infant in respiratory distress should be stimulated to cry, and an artificial oral airway may be used to avoid intubation. The clinical diagnosis is based on the inability to pass a small-caliber catheter through the nasal passages. However, the act of passing catheters, especially if repeated, may cause nasal passage swelling in any infant, and the subsequent iatrogenic occlusion can mimic the congenital condition.
An alternative noninvasive method of excluding the diagnosis of complete atresia is to place a glass slide under the nasal orifices and look for fogging with expiration. Supplemental oxygen should be administered to infants with choanal atresia, and an oral airway may be of assistance. If the infant remains in significant respiratory distress, intubation is necessary. Intubation relieves the obstruction so that little if any ventilation will be required.
Pierre Robin syndrome
Pierre Robin syndrome presents with micrognathia and resultant displacement of the (normal) tongue into the posterior pharynx, which may occlude the upper airway. A central cleft of the soft palate is usually present. Respiratory distress and cyanosis are caused by the obstruction of the upper airway.
In the delivery room, the infant should be given supplemental oxygen and placed in a prone position in an attempt to induce the tongue to move forward in a dependent fashion from the posterior pharynx and thereby relieve the airway obstruction. If the infant continues to have persistent respiratory distress, an oral airway may be placed.
Alternatively, an appropriately sized endotracheal (ET) tube may be passed through the nose into the hypopharynx. Tracheotomies are generally not necessary and should be avoided. Intubation of these infants often is not easy, because visualization of the larynx is difficult.
Tracheal webbing
The pathogenesis of tracheal webbing originates in the 10th week of gestation, when an arrest in the development of the larynx near the vocal cords results in a residual web of tissue persisting in the airway. Approximately 75% of tracheal webs occur at the level of the vocal cords. These lesions are critical if more than 50% of the airway diameter is occluded, but this degree of occlusion is exceedingly rare. Tracheal webs may be relatively asymptomatic at birth, with the development of distress later when activity increases and the need for airway flow increases.
During attempted intubation, an obstructive covering may be observed over the larynx and may occlude the airway completely. If this membrane is thin, the ET tube may be pushed beyond the obstruction. If the membrane is thick, the infant requires an emergency tracheotomy. If the infant is in severe distress, a large-bore needle or catheter may be placed in the trachea to allow gas exchange while emergency treatment is being arranged. Inexperienced personnel may confuse this rare disorder with simple inability to visualize the vocal cords.
Esophageal atresia with or without tracheoesophageal fistula
Esophageal atresia is rarely considered a life-threatening emergency; however, early diagnosis is essential to prevent further complication. It may be divided into 5 types as follows:
-
Type I (esophageal atresia with a distal fistula) - This is the most common type (85%); air is present in the stomach; a blind upper esophageal segment is present, with the distal segment of the esophagus connected to the trachea via a fistula
-
Type II (esophageal atresia only) - A blind upper and lower esophageal segment is present; air is absent from the lower gastrointestinal (GI) tract, but an air-filled blind upper pouch may be observed
-
Type III (H-type esophageal atresia) - An isolated fistula connects the esophagus and trachea, usually occurring at the upper portion of the trachea and esophagus
-
Type IV (esophageal atresia with a proximal fistula) - This type is rare; an upper esophageal segment is present with a fistula to the trachea and a blind lower esophageal segment; air is absent from the lower GI tract
-
Type V (esophageal atresia with a double fistula) - This type is rare; an upper esophageal segment is present with a fistula to the trachea, and a second fistula connects the distal esophagus and trachea; air is present in the stomach
The most common clinical symptoms of esophageal atresia with or without a tracheoesophageal fistula include coughing, choking, and cyanosis. Infants with isolated esophageal atresia usually do not demonstrate respiratory distress immediately in the delivery room but may have excess secretions. The atretic air-filled esophageal pouch occasionally may be observed on a chest radiograph, manifested by a midthoracic rounded lucency. This pouch is visualized more readily by the passage of a radiopaque catheter into the esophagus before the chest radiograph.
Because secretions or oral feedings cannot pass into the stomach, the contents of the esophageal pouch readily reflux, placing these infants at high risk for aspiration. A Replogle suction catheter should be inserted to reach the esophageal pouch and placed on low continuous suction as soon as possible. Infants with an associated distal fistula to the trachea are also at high risk for aspiration of gastric contents into the lungs via the gastrobronchial fistula, which most often empties into the airway near the carina.
If at all possible, delivery of any positive-pressure ventilation (PPV) or PEEP (ie. CPAP) should be avoided in these infants. Any positive pressure applied to the airway results in inflation of the fistula, stomach, and bowel, which then results in abdominal distention. This distending pressure cannot be relieved by esophageal reflux through the atretic esophagus. Relief of the distending pressure occurs with reflux of gastric contents into the lungs via the fistula. The continued application of PPV also may lead to massive gastric distention and possible rupture.
In rare emergency situations, percutaneous gastrotomy may be required to decompress the stomach; however, controlled surgical placement of a gastrostomy tube is preferable.
Cystic adenomatoid malformation
Cystic adenomatoid malformations of the lung are masses that may cause a spectrum of symptoms, from massive mediastinal shifts in the fetus (resulting in pulmonary hypoplasia) to isolated subsegmental lobar masses in the newborn (or adult) with minimal associated symptoms. Severe lesions also may cause fetal cardiac compromise and result in hydrops.
If the infant requires PPV, extreme caution must be used, because the distending pressure may inflate the cystic malformation. An inflated cystic malformation is capable of massive expansion, causing respiratory embarrassment because of the prevention of ventilation of other normal lung tissue.
Cystic hygromas
Cystic hygroma is the result of a congenital deformity of the lymphatic channels. Lymph accumulates and may compress the airway, depending on the size and location of the lymph accumulation. Approximately 80% of these lymphatic cystic accumulations occur in the neck and may in rare cases compress the trachea.
These infants may present with significant respiratory distress and may require immediate intubation with deep positioning of the ET tube to relieve the obstruction by stenting open the airway. However, most of these lesions expand outward from the neck and do not cause significant airway compromise in the delivery room.
Pulmonary compression
Congenital diaphragmatic hernia
The pathogenesis of congenital diaphragmatic hernia involves the incomplete formation of the diaphragm in the fetus, resulting in a migration of the abdominal viscera into the chest during development. If the defect is large and the abdominal viscera have caused long-standing compression of the developing lungs, pulmonary hypoplasia may develop.
The diagnosis of diaphragmatic hernia is frequently established by means of prenatal ultrasonography, which allows management to be transferred to a perinatal referral center where pediatric surgery and appropriate medical support, including extracorporeal bypass, are available. In the delivery room, the infant often presents with respiratory distress. Physical signs may include a scaphoid abdomen and a shift in heart sounds to the right hemithorax.
Respiratory distress in the delivery room may be caused by pulmonary hypoplasia or may be secondary to expansion of the bowel caused by swallowed air. The expansion of the bowel results in compression of the lung, much like the pathophysiology of a tension pneumothorax.
Delivery room management includes immediate intubation and passage of a large catheter for gastric decompression. Intubation prevents distention of the stomach and bowel contents because of crying or bag-valve-mask ventilation. The gastric decompression should be achieved with a Replogle or Salem pump suction catheter connected to a low continuous drain. Constant maintenance of gastric suction during the preoperative and immediate postoperative periods is essential. New modes of ventilation, such as high-frequency oscillatory ventilation (HFOV), have decreased the use of extracorporeal membrane oxygenation (ECMO) in this population but ECMO still may be required for the most severe of these lesions.
Pneumothorax and pneumomediastinum
Air leak syndromes are disorders produced when a rupture of pulmonary tissue occurs, leading to the escape of air into spaces where air would not normally be present. The incidence of pneumothorax varies with gestational age, severity of pulmonary disease, need for assisted ventilation, mode of ventilation, and expertise of delivery room personnel.
After the initial rupture of a small airway or an alveolus, air may enter the perivascular and peribronchial spaces and track along the lymphatic channels. Air that dissects into the hilum results in a pneumomediastinum; air that tracks into the pleural space manifests as a pneumothorax. Spontaneous rupture of the lung directly into the pleural space is thought to occur rarely but may be caused iatrogenically by the percutaneous insertion of a chest tube. Caution is required.
Pneumomediastinum frequently is an isolated disorder that occurs spontaneously in infants with minimal pulmonary disease. These infants usually are asymptomatic or minimally symptomatic because the air in the mediastinum can escape to the tissues of the neck. Intrathoracic tension is relieved, and circulation is not compromised. Infants with a pneumomediastinum should be observed. Intervention usually is unnecessary.
Pneumothorax may occur immediately in the delivery room or later, when significant pulmonary disease has developed. Pneumothorax often is associated with PPV, but it also may occur in infants who are not receiving assisted ventilation. After the initial air leak, the subsequent expansion of intrathoracic spaces often results in a rapid increase of intrathoracic pressure to the point where the lungs cannot be ventilated and venous blood cannot be returned to the heart. This condition is termed a tension pneumothorax.
The rapid clinical deterioration of infants with this condition is caused by circulatory collapse and inability to ventilate. Any infant who has a sudden precipitous change in ventilatory status associated with an abrupt fall in blood pressure should immediately be evaluated for a pneumothorax. Transillumination of the chest may be used for the rapid diagnosis of severe tension pneumothorax. When the clinical situation allows it, radiography should be performed to establish or confirm the diagnosis.
Infants in acute distress should undergo needle aspiration to evacuate the extrapulmonary air while preparations are made to place a chest tube. Symptomatic pneumothorax is managed with the insertion of a chest tube until the pulmonary leak is resolved. A chest tube may not be required if the pneumothorax is small and the infant is not receiving PPV. Supplemental oxygen (fraction of inspired oxygen (FI O2) of 0.4 -1.0) often is administered for 6-12 hours to hasten reabsorption of the trapped intrapleural air.
Miscellaneous conditions
Multiple gestation
The delivery and subsequent resuscitation of multiple infants presents a considerable challenge to the labor and delivery team. The first consideration to be addressed with the initial prenatal diagnosis of multiple births is to ensure that care is being provided at an institution capable of adequately supporting both the mother and multiple infants at delivery, often with minimal notice.
A minimum of 2 experienced staff members should be available for each infant. Multiple-gestation infants are often born prematurely (especially with higher-order gestation), and more personnel may be required for each infant. Therefore, for higher-order gestation involving triplets or more, preparation to ensure the presence of appropriate personnel and equipment must be planned well in advance of the delivery.
The team should be led and organized by a designated experienced leader, and the planning phase should involve healthcare providers from a number of disciplines, including neonatologists, perinatologists, nurse practitioners, pediatricians, nursery and obstetrics nurses, respiratory therapists, and pharmacists. The delivery team should consist of individuals who are prepared to make complex decisions, perform technical skills, and respond quickly to changing circumstances.
Organization and teamwork are essential, with adequate personnel prospectively identified as designated responders to each infant. Such preparations are becoming more commonplace, now that assisted conception is giving rise to an increasing number of multiple-birth pregnancies.
Hydrops
When preparing for the resuscitation of an infant with hydrops fetalis, sufficient skilled personnel must be in the delivery room to ensure that the multiple needs of this significantly compromised neonate can be met. Equipment should be prepared before the delivery, and all personnel in the room should be assigned specific procedures, such as paracentesis or thoracentesis (if required). These procedures may have to be performed immediately if the fluid accumulation is causing difficulties in ventilation.
If the hydrops is caused by anemia, blood for transfusion should be available in the delivery room. Because of the potential for excess fluid in the chest, it is often necessary to use high pressures and oxygen initially. Artificial surfactant administration also has been employed in the delivery room to treat any surfactant deficiency in an attempt to improve pulmonary function. Umbilical venous and arterial lines should be placed and central venous pressures monitored.
Omphalocele and gastroschisis
Gastroschisis is an abdominal wall defect lateral to the umbilicus that does not have a sac or membrane covering the bowel. In contrast, an omphalocele involves herniation of the bowel through the umbilical opening, with the bowel covered by a thin membrane, unless the membrane has been ruptured during birth.
For both omphalocele and gastroschisis, maintain adequate intravascular fluid volume, maintain thermoregulation, and prevent bowel ischemia. Preoperatively, infants with these conditions have increased fluid requirements unless the bowel is appropriately wrapped with an airtight material (a "bowel bag").
The bowel may be first wrapped with warmed saline-soaked gauze. Care should be taken to support the bowel and not compromise blood flow. Observe the bowel closely to ensure that no areas are compromised as a result of the bowel twisting. A 10 French Replogle or Salem pump suction catheter should be placed on low continuous suction to decompress the bowel and prevent further ischemic injury.
If the infant is diagnosed with an omphalocele, the blood glucose level should be assessed because this defect may be associated with Beckwith-Wiedemann syndrome. Trisomy 18 is associated with this anomaly. Therefore, if the condition is recognized prenatally, amniocentesis for chromosomal analysis should be offered to the family. If chromosomal information is not available at the time of delivery and there are other anomalies consistent with trisomy 18, surgery should be delayed until a complete genetic evaluation is complete.
Congenital anomalies
Severe malformations observed in the delivery room should not alter resuscitative management unless skilled and experienced care providers are able to determine that the condition is incompatible with life. If possible, the family should be involved in any decision involving the withholding of resuscitation. Ideally, infants with malformations that have not been diagnosed prenatally should be resuscitated and stabilized until an accurate diagnosis can be made and discussions held with the family.
Controversies in Resuscitation
The development of a certification program has led to the standardization of neonatal resuscitation. Evaluation of the current standards is an ongoing process. As new research is published, it is essential to assess the quality of the studies and to determine whether the evidence is sufficient to mandate changes in practice. Even with the current standardization, there remain some important controversies and concerns in resuscitation.
Room air versus 100% oxygen
Oxygen is a drug with potentially serious adverse effects that must be considered. Oxygen free radicals are capable of tissue injury and have been implicated in several disease states in the neonate. The use of lower oxygen concentrations in neonatal resuscitation may decrease the number of oxygen free radicals and their damaging adverse effects.
In one study, resuscitation with room air was shown to be as effective as 100% oxygen at lowering pulmonary vascular resistance. Other investigations have shown that there is no benefit to raising the partial pressure of oxygen (PO2) above 50 mm Hg. A 2011 meta-analysis concluded that the literature was insufficient to support any statement regarding the superiority of oxygen or room air as the initial gas mixture for neonatal resuscitation. [12]
Although large controlled multicenter trials indicate that room air (fraction of inspired oxygen [FI O2) = 0.21) is just as effective as 100% oxygen in resuscitating term infants, long-term outcomes are pending. The only follow-up study looking at these infants at 18-24 months showed no significant difference in somatic growth or neurologic handicaps between infants resuscitated in room air and infants receiving 100% oxygen.
Timing of artificial surfactant administration
Surfactant deficiency, the primary factor in the development of respiratory distress syndrome (RDS), is the most common cause of persistent and progressive respiratory distress in premature infants.
Controlled, randomized clinical studies have shown that prophylactic administration of exogenous surfactant to premature infants effectively reduces death secondary to RDS. Studies have also shown that treatment of only infants who develop RDS symptoms yields a significant reduction in death secondary to RDS. Prophylactic dosing of artificial surfactant is performed in the delivery room before the first breath or within 15 minutes after birth.
Controversies related to prophylactic artificial surfactant use are related to the interruption of the standard resuscitation paradigm for the administration, treatment, and attendant risk management of a population of infants who would not develop RDS, as well as the potential additional costs related to this dosing scheme.
The argument for prophylactic surfactant dosing is that treated infants who require surfactant replacement have more uniform and effective drug distribution when the lungs are fluid-filled and do not have air-fluid interfaces. Obviously, treatment of only infants with a confirmed diagnosis of RDS results in a smaller number of infants being treated. The proportion of infants given prophylactic artificial surfactant therapy who would not develop RDS depends on the entry criteria for prophylactic treatment and on population characteristics.
Studies have demonstrated that early prophylactic dosing of surfactant is efficacious and is associated with better outcomes in extremely premature infants. Some researchers have recommended that, whenever possible, infants with surfactant deficiency should be identified before delivery by using the lecithin-sphingomyelin ratio or testing for the presence of phosphatidylglycerol.
Researchers also suggest that all infants delivered earlier than 26-28 weeks' gestation should receive their first dose of surfactant in the delivery room within the first few minutes of life, after cardiopulmonary stabilization. InfantsProgram had recommended endotracheal suctioning of all non-vigorous infants born later than 30 weeks' gestation should receive rescue therapy as soon as they show clinical signs of RDS. Infants born at 30-36 weeks' gestation may benefit from surfactant with rapid extubation to continuous positive airway pressure (CPAP). [13, 14, 15, 16]
Intubation and suctioning for meconium aspiration
Previously, the NRP Program had recommended endotracheal suctioning of all non-vigorous infants with meconium-stained fluid, however this was based on small studies and largely historical precedent and is now no longer recommended.
However, trained personnel should be present for infants born through meconium-stained fluid. Meconium-stained amniotic fluid (MSAF) is present in 10-15% of all deliveries. Of newborns born through MSAF, 60% require some interventions for stabilization or resuscitation. Of these 60%, 3-4% are subsequently diagnosed with meconium aspiration syndrome (MAS). Meconium aspiration in a newborn can lead to atelectasis, overdistention of the alveoli, pneumothorax, pneumonitis, surfactant deficiency, and persistent pulmonary hypertension. Mortality is as high as 5-10% in these infants. [17]
Routine suctioning of the oropharynx and nasal pharynx once the head is delivered is also no longer recommended. A large, multicenter, randomized trial with 2514 infants showed that intrapartum suctioning did not decrease the risk of MAS. The current Neonatal Resuscitation program (NRP) guidelines and recommendations no longer advise routine intrapartum oropharyngeal and nasopharyngeal suctioning for infants born to mothers with MSAF. [18] Therefore, a bulb syringe should not be used by the obstetric personnel routinely for every infant, but should be utilized by the neonatal resuscitation personnel to clear the nose and mouth of thick meconium.
Hypothermia
Current data are insufficient to support recommendation of systemic or head cooling for infants with suspected asphyxia. Study results are conflicting. One multicenter trial did not show a difference in the number of survivors with severe disabilities when head cooling was used. Another large multicenter trial that evaluated systemic hypothermia found a significant decrease in death or moderate disability at age 12 months and 18 months, as did a smaller trial.
Hypothermia carries risks of arrhythmias, bleeding, thrombosis, and sepsis; however, current studies of modest, controlled hypothermia have not reported these complications. Future clinical trials are needed to determine the benefits of hypothermia and to compare methods of cooling. Avoiding hyperthermia in infants who have suffered a hypoxic-ischemic event at birth is essential. Studies have shown that hyperthermia of 2-3° can worsen outcome.
Withholding and discontinuing resuscitation
Neonatal morbidity and mortality vary throughout the United States. The obstetric and neonatal team, in conjunction with the parents, should decide whether and when to withhold or discontinue resuscitative efforts. Infants whose gestational age, birth weight, and congenital anomalies are associated with certain death should not be resuscitated. These may include infants with extreme prematurity (< 23 weeks' gestation), extremely low birth weight (< 400 g) or chromosomal anomalies inconsistent with life (eg, Trisomy 13).
In other situations where the prognosis is uncertain but the associated morbidity is high, parental desires should be considered. Discontinuing resuscitation may be justified in infants who have not responded to continuous and appropriate resuscitation for a full 10 minutes and who have no heart rate or respiratory effort (ie, no signs of life).
Guidelines
American Heart Association
In 2020, the American Heart Association released their updated recommendations for neonatal resuscitation. [19]
Cord management
Clamping of the umbilical cord may be delayed for more than 30 seconds in term and preterm infants after an uncomplicated birth. Note that umbilical cord milking is not recommended for preterm infants.
Prevention of hypothermia
Skin-to-skin contact with the mother is recommended for healthy newborn infants because it can promote breastfeeding, improve blood glucose stability, and help prevent hypothermia. The infant’s temperature should be maintained between 97.7°F (36.5°C) and 99.5°F (37.5°C).
Tactile stimulation
If a newborn infant is breathing ineffectively or has apnea, drying the infant and/or rubbing the back and soles of the feet may help stimulate breathing.
Clearing the airway
Routine oral, nasal, oropharyngeal, or endotracheal suctioning is not recommended for newborn infants, even those who are born with born with meconium-stained amniotic fluid (MSAF). However, nonvigorous infants with MSAF at birth who have evidence of airway obstruction can benefit from intubation and tracheal suction.
Ventilatory support
Start positive-pressure ventilation (PPV) without delay in newborn infants who are gasping or apneic within 60 seconds after birth or who have persistent bradycardia (heart rate of < 100 beats/min). A rate of 40 to 60 inflations per minute is reasonable. A key indicator of successful ventilation is an increase in heart rate.
Oxygen therapy
PPV may be started with air (21% oxygen) in term and late preterm infants; up to 30% oxygen may be used in preterm infants (less than 35 weeks’ gestation). The use of 100% oxygen should be avoided in term and late preterm newborns because it is associated with excess mortality.
Heart rate assessment
Electrocardiography can provide rapid and accurate measurement of the heart rate during the resuscitation of term and preterm newborn infants.
Chest compressions
Initiate chest compressions if the heart rate is lower than 60 beats/min after at least 30 seconds of adequate PPV.
Intravascular access
The umbilical vein is the recommended route for vascular access in infants who have failed to respond to PPV and chest compressions and who require epinephrine and/or volume expanders.
Epinephrine administration
Administer epinephrine, preferably intravenously, if the heart rate remains lower than 60 beats/min despite 60 seconds of chest compressions and adequate PPV. The recommended intravenous dose of epinephrine is 0.01 to 0.03 mg/kg.
Volume expansion
Failure to respond to epinephrine and known or suspected blood loss are indications for volume expansion with normal saline or blood. The recommended initial volume is 10 mL/kg over 5 to 10 minutes.
Care after resuscitation
Newborn infants who received prolonged PPV, intubation, chest compressions, or epinephrine should be monitored closely in a neonatal intensive care unit or similar area after their condition has stabilized.
-
Fetal response to asphyxia illustrating initial redistribution of blood flow to vital organs. With prolonged asphyxial insult and failure of compensatory mechanisms, cerebral blood flow falls, leading to ischemic brain injury.
-
Assisted ventilation newborn –Intubation and meconium aspiration. Video courtesy of Therese Canares, MD, and Jonathan Valente, MD, Rhode Island Hospital, Brown University.






